The rate of a certain reaction was studied at various T(K) k (s-1) temperatures. The table shows temperature (T) and rate 400 0.0000173 constant (k) data collected during the experiments. Plot the 420 0.000127 data to answer the questions. What is the value of the activation energy, Ea, for 440 0.000773 460 0.00403 this reaction? 480 0.0183 500 0.0738 Ea = kJ · mol-! 520 0.267 540 0.879 560 2.66 What is the value of the pre-exponential factor (sometimes 580 7.43 called the frequency factor), A , for this reaction? A = s-1
The rate of a certain reaction was studied at various T(K) k (s-1) temperatures. The table shows temperature (T) and rate 400 0.0000173 constant (k) data collected during the experiments. Plot the 420 0.000127 data to answer the questions. What is the value of the activation energy, Ea, for 440 0.000773 460 0.00403 this reaction? 480 0.0183 500 0.0738 Ea = kJ · mol-! 520 0.267 540 0.879 560 2.66 What is the value of the pre-exponential factor (sometimes 580 7.43 called the frequency factor), A , for this reaction? A = s-1
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter11: Chemical Kinetics
Section: Chapter Questions
Problem 98CWP: A certain reaction has the form aAProducts At a particular temperature, concentration versus time...
Related questions
Question
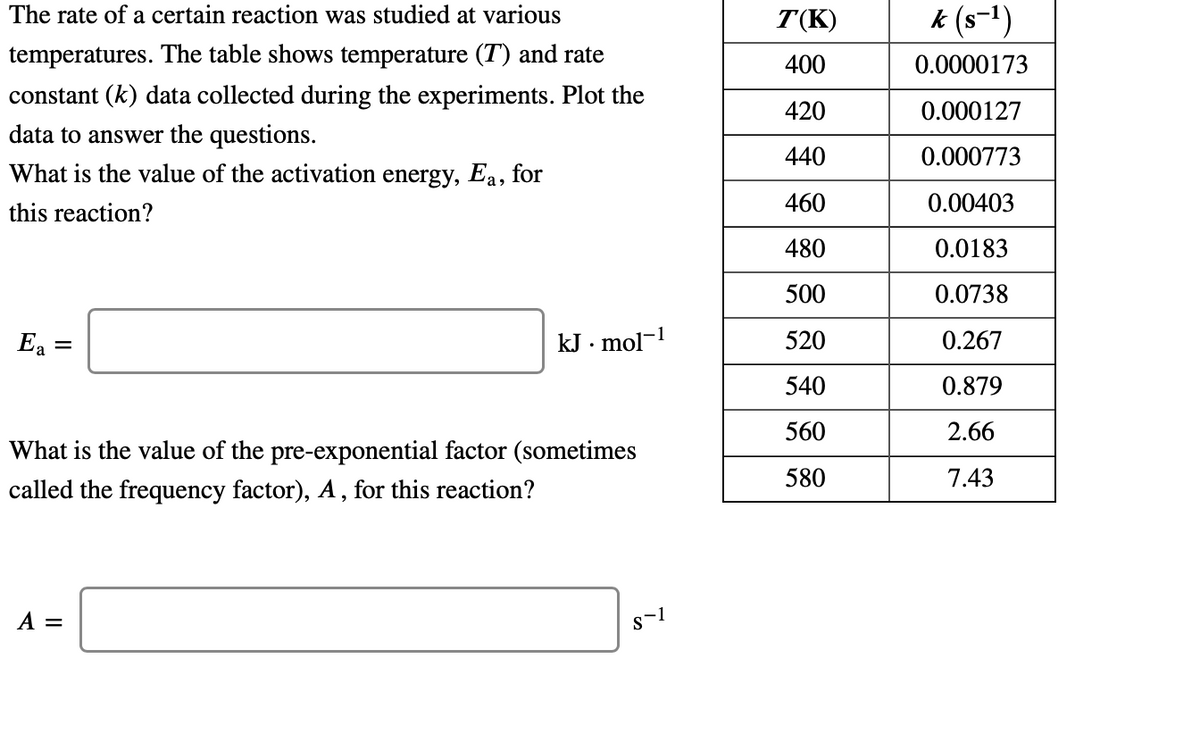
Transcribed Image Text:The rate of a certain reaction was studied at various
T(K)
k (s-1)
temperatures. The table shows temperature (T) and rate
400
0.0000173
constant (k) data collected during the experiments. Plot the
420
0.000127
data to answer the questions.
What is the value of the activation energy, Ea, for
440
0.000773
460
0.00403
this reaction?
480
0.0183
500
0.0738
Ea
kJ · mol-1
520
0.267
540
0.879
560
2.66
What is the value of the pre-exponential factor (sometimes
580
7.43
called the frequency factor), A , for this reaction?
A =
s-1
Expert Solution
Step 1
The expression of Arrhenius equation is shown below:
Where;
k = rate constant
A = Arrhenius constant
Ea = activation energy
R = gas constant
T = temperature
Trending now
This is a popular solution!
Step by step
Solved in 6 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,