us, the observed eléctron configuration of the Cr atom is 1s 2s 2p" 3s Part D Mo has an anomalous electron configuration. Write the observed electron configuration of Mo. Express your answer in complete form in order of orbital filling. For example, 1s 2s should be entered as 1s^22s^2. > View Available Hint(s) Submit Previous Answers 77°F Partly cloudy A
us, the observed eléctron configuration of the Cr atom is 1s 2s 2p" 3s Part D Mo has an anomalous electron configuration. Write the observed electron configuration of Mo. Express your answer in complete form in order of orbital filling. For example, 1s 2s should be entered as 1s^22s^2. > View Available Hint(s) Submit Previous Answers 77°F Partly cloudy A
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter12: Atoms And Molecules
Section: Chapter Questions
Problem 12.1E: In the Stern-Gerlach experiment, silver atoms were used. This was a good choice, as it turned out....
Related questions
Question
Transcribed Image Text:Chapter 3, Part 1 homework
Animation-Electron Configurations
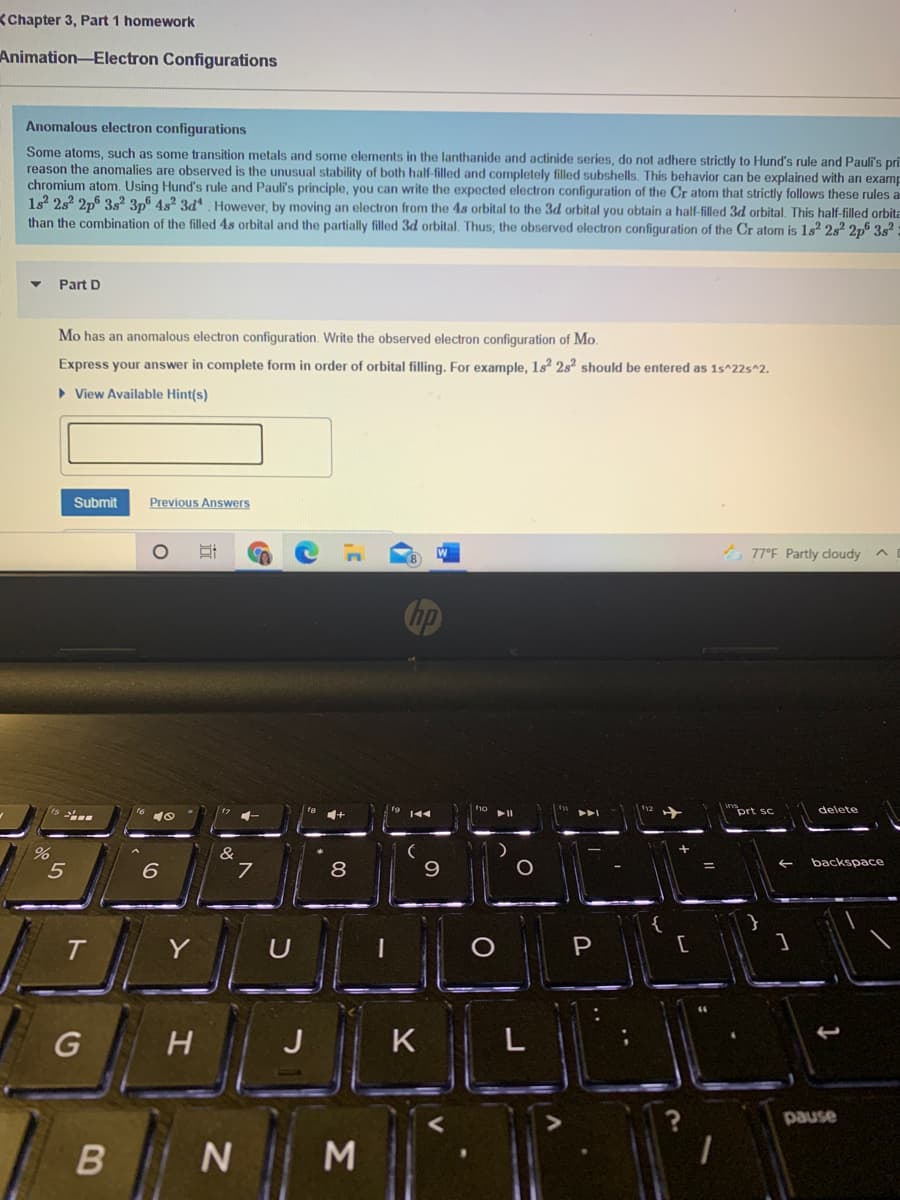
Anomalous electron configurations
Some atoms, such as some transition metals and some elements in the lanthanide and actinide series, do not adhere strictly to Hund's rule and Pauli's pri
reason the anomalies are observed is the unusual stability of both half-filled and completely filled subshells. This behavior can be explained with an examp
chromium atom. Using Hund's rule and Pauli's principle, you can write the expected electron configuration of the Cr atom that strictly follows these rules a
1s2 2s? 2p6 3s2 3p 4s2 3d However, by moving an electron from the 4s orbital to the 3d orbital you obtain a half-filled 3d orbital. This half-filled orbita
than the combination of the filled 4s orbital and the partially filled 3d orbital. Thus; the observed electron configuration of the Cr aton is 1s² 2s² 2p® 3s²
Part D
Mo has an anomalous electron configuration. Write the observed electron configuration of Mo.
Express your answer in complete form in order of orbital filling. For example, 1s² 2s should be entered as 1s^225^2.
> View Available Hint(s)
Submit
Previous Answers
o 77°F Partly cloudy
Chp
prt sc
delete
&
backspace
6.
8
T
Y
U
G H
J K
pause
B N M
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co