
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
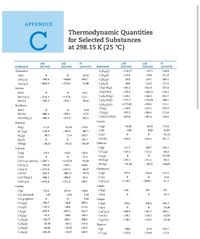
Transcribed Image Text:APPENDIX
Thermodynamic Quantities
for Selected Substances
at 298.15 K (25 °C)
AH
(ki/mol)
AG?
(k/mol)
AH
(kj/mol)
AG
(kj/mol)
S°
Substance
(1/mol-K)
Substance
(1/mol-K)
Aluminum
CHulg)
-124.73
- 15.71
310.0
Al(s)
28.32
-1476
-15.0
231.0
AICH (1)
-705.6
-630.0
109.3
CH, (g)
82.9
129.7
269.2
- 1669.8
- 1576.5
51.00
CH, (1)
CHOH(g)
49.0
124.5
172.8
Barium
-201.2
- 161.9
237.6
CH,OH(I)
CH,OH(g)
C,H,OH(I)
CH06(s)
Co(g)
CO(g)
CH,COOH(I)
-238.6
- 166.23
126.8
Ba(s)
63.2
-235.1
- 168.5
282.7
BaCO,(s)
BaO(9)
- 1216.3
-1137.6
112.1
-553.5
-525.1
70.42
-277.7
- 174.76
160.7
-1273.02
-910.4
212.1
Beryllium
-110.5
-137.2
197.9
Be(s)
9.44
-393.5
-394.4
213.6
BeO(s)
-608.4
-579.1
13.77
-4870
-392.4
159.8
Be(OH),(4)
-905.8
-817.9
50.21
Cesium
Bromine
Cslg)
76.50
49.53
175.6
Brg)
Br" (aq)
Bry (g)
Bry (1)
HBr(g)
Calcium
111.8
82.38
174.9
- 120.9
- 102.8
80.71
Cs(f)
2.09
0.03
92.07
30.71
3.14
245.3
Cs(s)
85.15
152.3
CSCI()
-442.8
-414.4
101.2
-36.23
-53.22
198.49
Chlorine
121.7
105.7
165.2
179.3
cr(ag)
-167.2
-131.2
56.5
Calg)
Ca(s)
145.5
154.8
222.96
41.4
CaCO,(s, calcite) -1207.1
HCI(ag)
-167.2
-131.2
56.5
-1128.76
92.88
HCI(g)
-92.30
-95.27
186.69
CaCl,(s)
CaF,(s)
-795.8
-748.1
104.6
- 1219.6
-1167.3
68.87
Chromium
CaO()
-635.5
-604.17
39.75
Cre)
397.5
352.6
174.2
Ca(OH),(s)
CasO(s)
-986.2
- 898.5
83.4
Cr(s)
23.6
- 1434.0
- 1321.8
106.7
-1139.7
-1058.1
81.2
Carbon
Cobalt
718.4
672.9
158.0
Colg
439
393
179
C(s, diamond)
1.88
284
Co(s)
2.43
28.4
C(s. graphite)
5.69
Copper
Culg)
- 106.7
-64.0
309.4
338.4
298.6
166.3
CCL(1)
CF,(g)
CH (g)
C,H(g)
C,H(g)
- 139.3
-68.6
214.4
Cu(s)
33.30
-679.9
-635.1
262.3
CuCl,()
CuO)
-205.9
- 161.7
108.1
-74.8
-50.8
186.3
-156.1
- 128.3
42.59
226.77
209.2
200.8
Cu,O(s)
-170.7
-147.9
92.36
52.30
68.11
219.4
Fluorine
-84.68
-32.89
229.5
158.7
F(g)
F"(aq)
80.0
61.9
C,H(g)
- 103.85
-23.47
269.9
-332.6
-278.8
- 13.8

Transcribed Image Text:5) Using values from Appendix C, calculate the standard enthalpy change for each
of the following reactions:
(a) 2 SO2(g) + O2(8)
2 SO3(8)
(b) Mg(OH)2(s)
MgO(s) + H2O(1)
(c) N2O4(8) + 4 H2(g)
→ N2(8) + 4 H2O(g)
(d) SiCl4(1) + 2 H2O(I)
SiO2(s) + 4 HCl(8)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A sample of a hydrocarbon is combusted completely inO2(g) to produce 21.83 g CO2(g), 4.47 g H2O(g), and 311 kJ ofheat. (a) What is the mass of the hydrocarbon sample thatwas combusted? (b) What is the empirical formula of thehydrocarbon? (c) Calculate the value of ΔHf° per empirical formulaunit of the hydrocarbon. (d) Do you think that thehydrocarbon is one of those listed in Appendix C? Explainyour answer.arrow_forward2 (a) In this investigation, you will use a polystyrene-cup calorimter to determine the amount of heat released during acid-base neutralization reactions. Find information in your textbook, an encyclopedia, or a web site about calorimetry. Sketch the apparatus for a polystrene-cup calorimeter. (b) For more precise heat of reaction measurnments, chemists use a device called bomb calorimeter. How is a bomb calorimeter similar to and different from a polystyrene-cup calorimeter?arrow_forwardWhich of the following reactions corresponds to the standard enthalpy of formation of methane CH4? A: C(s, gr) + 2H2(g) —> CH4(g) B; CH4(g) —> C(s, gr) + 2H2(g) C: C(s,gr) + 4H (g) —> CH4 D:CH4–> C(s,gr) + 4H(g) Choose one. Thanks!arrow_forward
- For reaction (b) HCl(g) + NaOH(s) NaCl(s) + H20(I), what is the equation for the change in enthalpy of this reaction? O [AHf° (NaCI(s)) + AHf°(H20(1)] - [AHP(HCI(g)) + AHP(N2OH(s))] O [AHP (HCI(g)) + AHP(N2OH(s))] - [AHP(NaCI(s)) + AHP(H20(1))] Click if you would like to Show Work for this question: Open Show Work SHOW HINT SHOW SOLUTION Part 4 X Your answer is incorrect. Try again. Use the values from the given table and the equation you chose in Part 3 to find the overall change in enthalpy for the reaction. -55200arrow_forwardGiven the standard enthalpy changes for the following two reactions: ΔΗ° (1) N₂(g) + 2O2 (g) → 2NO2 (g) (2) 2N₂O(g) → 2N2(g) + O2(g) ΔΗ° = 66.4 kJ Standard enthalpy change : = - 164.2 kJ What is the standard enthalpy change for the following reaction? (3) 2N₂O(g) + 3O2 (g) → 4NO2 (g) AH° =? kJarrow_forwardConsider the following reaction. CH3OH(g) CO(g) + 2 H2(g) H = +90.7 kJ (a) Is the reaction exothermic or endothermic? endothermicexothermic (b) Calculate the amount of heat transferred when 40.0 g of CH3OH(g) are decomposed by this reaction at constant pressure.H = kJ(c) If the enthalpy change is 11.0 kJ, how many grams of hydrogen gas are produced? g(d) How many kilojoules of heat are released when 13.5 g of CO(g) reacts completely with H2(g) to form CH3OH(g) at constant pressure?H = kJ(e) Calculate E when 360.0 g of CH3OH(g) completely reacts at a constant temperature of 300 K and constant pressure of 0.95 atm. R = 8.314 J/mol*K and R = 0.08206 atm*L/mol*K kJ HopHelpCh5N1arrow_forward
- Consider the following reaction. CH3OH(g) CO(g) + 2 H2(g) H = +90.7 kJ (a) Is the reaction exothermic or endothermic? exothermic endothermic (b) Calculate the amount of heat transferred when 55.0 g of CH3OH(g) are decomposed by this reaction at constant pressure.H = kJ(c) If the enthalpy change is 11.0 kJ, how many grams of hydrogen gas are produced? g(d) How many kilojoules of heat are released when 12.0 g of CO(g) reacts completely with H2(g) to form CH3OH(g) at constant pressure?H = kJ(e) Calculate E when 450.0 g of CH3OH(g) completely reacts at a constant temperature of 300 K and constant pressure of 0.95 atm. R = 8.314 J/mol*K and R = 0.08206 atm*L/mol*Karrow_forwardConsider the following reaction. CH3OH(g) CO(g) + 2 H2(g) H = +90.7 kJ (a) Is the reaction exothermic or endothermic? endothermicexothermic (b) Calculate the amount of heat transferred when 40.0 g of CH3OH(g) are decomposed by this reaction at constant pressure.H = kJ(c) If the enthalpy change is 11.0 kJ, how many grams of hydrogen gas are produced? g(d) How many kilojoules of heat are released when 13.5 g of CO(g) reacts completely with H2(g) to form CH3OH(g) at constant pressure?H = kJ(e) Calculate E when 360.0 g of CH3OH(g) completely reacts at a constant temperature of 300 K and constant pressure of 0.95 atm. R = 8.314 J/mol*K and R = 0.08206 atm*L/mol*K kJ HopHelpCh5N1arrow_forwardWhen 0.638 of sodium metal is added to an excess of hydrochloric acid, 6630j of heat are produced. What is the enthalpy of the reaction as written? 2Na(s)+2HCl(aq)⟶2NaCl(aq)+H2(g) Enthalpy of reaction:arrow_forward
- Use Hess’s law to calculate the enthalpy change for the reaction WO3(s) + 3H2(g) —> W(s) +3H2O(g) from the following data: 2W(s) + 3O2(g) —> 2 WO3(s) H=-1685.4 2H2(g) + O2(g) —> 2H2O(g) H=-477.84arrow_forward5(a)arrow_forwardWhen methanol, CH3OH,CH3OH, is burned in the presence of oxygen gas, O2,O2, a large amount of heat energy is released. For this reason, it is often used as a fuel in high performance racing cars. The combustion of methanol has the balanced, thermochemical equation CH3OH(g)+32O2(g)⟶CO2(g)+2H2O(l)ΔH=−764 kJCH3OH(g)+32O2(g)⟶CO2(g)+2H2O(l)ΔH=−764 kJ How much methanol, in grams, must be burned to produce 639 kJ639 kJ of heat?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY