Which of the following compounds are aromatic based on the principles of the Huckel rule of aromaticity? 1 2 3 4 OH O D. Molecules 2 and 4 O C. Molecules 1 and 4 O E. Molecules 3 and 4 O B. Molecules 1 and 3 O A. Molecules 1 and 2
Which of the following compounds are aromatic based on the principles of the Huckel rule of aromaticity? 1 2 3 4 OH O D. Molecules 2 and 4 O C. Molecules 1 and 4 O E. Molecules 3 and 4 O B. Molecules 1 and 3 O A. Molecules 1 and 2
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter21: Benzene And The Concept Of Aromaticity
Section: Chapter Questions
Problem 21.16P: Which of the molecules and ions given in Problem 21.15 are aromatic according to the Hckel criteria?...
Related questions
Question
Solve all parts otherwise I will downvote
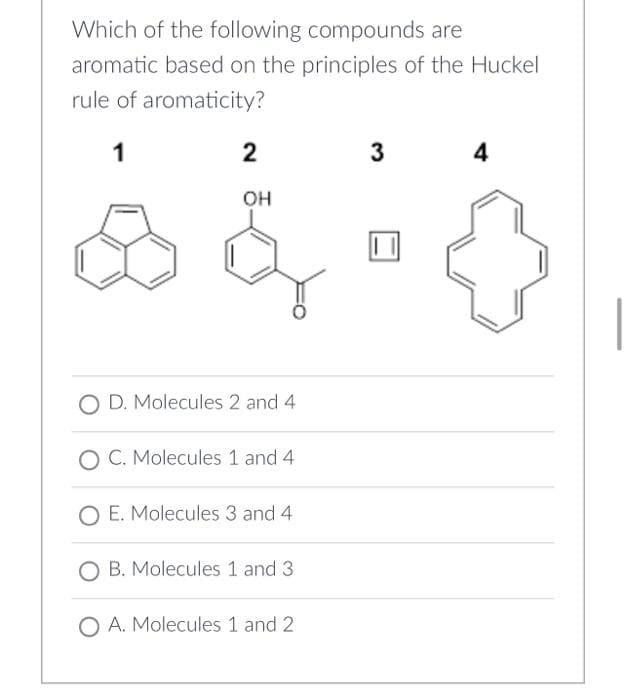
Transcribed Image Text:Which of the following compounds are
aromatic based on the principles of the Huckel
rule of aromaticity?
1
2
3
4
OH
O D. Molecules 2 and 4
O C. Molecules 1 and 4
O E. Molecules 3 and 4.
O B. Molecules 1 and 3
O A. Molecules 1 and 2
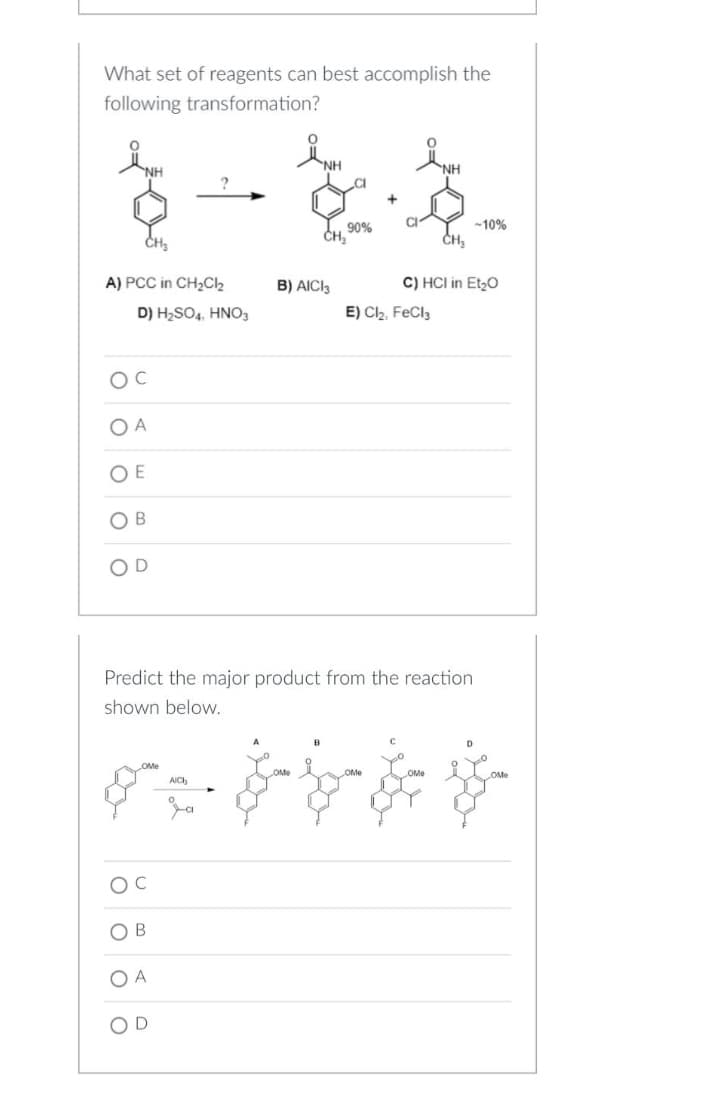
Transcribed Image Text:What set of reagents can best accomplish the
following transformation?
NH
NH
90%
A) PCC in CH₂Cl₂
D) H₂SO4, HNO3
CH₂
B) AICI 3
-10%
C) HCI in Et₂O
E) Cl₂, FeCl3
OC
OA
OE
OB
OD
Predict the major product from the reaction
shown below.
B
D
OMe
OMe
OMe
AICH
$.$-b- &
O C
OB
OA
OD
OMe
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning



Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


