Which set of atoms below are transition metals that have only one electron in their outermost "s" orbitals? 1 H Не 1.009 4.000 m ben ongen 3 4 5 6 10 Li Be C F Ne 14 shospten 15 16. 2010 s0122 magnestm 12 12:011 odum 16 17 18 11 13 14 Na Mg AI Si P CI Ar 24 220 potavm 19 va m 23 mongn 25 tro 35 orm bat pmantm knen 31 28 30 Cr Mn Fe Co Ni Cu Zn Ga Ge As Se 20 21 22 24 26 27 29 32 33 34 36 к Са Sc Ti V Br Kr 241 242 antony stontum 38 pallatm 46 odie 53 niem catmim Inm 50 In Sn Sb Te 37 39 40 41 42 43 44 45 47 48 49 51 52 54 Rb| Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd Хе sor hamm 72 126 astate 85 bortum tantam heum meroun Inad bemuth pokn aon 55 56 57-70 71 73 74 75 76 77 78 79 80 81 82 83 84 86 Cs Ba Lu Hf Ta W Re Os Ir Pt Au Hg TI Pb Bi Po At Rn 20 1830 mabiongam 106 boh 107 ranm 87 89-102 103 104 105 108 109 110 111 112 114 Fr Ra ** Lr Rf Db Sg Bh Hs Mt Uun Uuu Uub Uuq lathanm 57 62 63 hom 67 tham 69 "70 59 60 61 64 65 66 68 *Lanthanide series La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb 14 prd 91 144 174 **Actinide series 19 97 99 90 92 93 94 96 98 100 101 102 Ac Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No p psa Select one: O a. Zn, Cd, Hg O b. Cr, Mo, W O . K, Rb, Cs O d. Sc, Y, Lu O e. Mn, Tc, Re
Which set of atoms below are transition metals that have only one electron in their outermost "s" orbitals? 1 H Не 1.009 4.000 m ben ongen 3 4 5 6 10 Li Be C F Ne 14 shospten 15 16. 2010 s0122 magnestm 12 12:011 odum 16 17 18 11 13 14 Na Mg AI Si P CI Ar 24 220 potavm 19 va m 23 mongn 25 tro 35 orm bat pmantm knen 31 28 30 Cr Mn Fe Co Ni Cu Zn Ga Ge As Se 20 21 22 24 26 27 29 32 33 34 36 к Са Sc Ti V Br Kr 241 242 antony stontum 38 pallatm 46 odie 53 niem catmim Inm 50 In Sn Sb Te 37 39 40 41 42 43 44 45 47 48 49 51 52 54 Rb| Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd Хе sor hamm 72 126 astate 85 bortum tantam heum meroun Inad bemuth pokn aon 55 56 57-70 71 73 74 75 76 77 78 79 80 81 82 83 84 86 Cs Ba Lu Hf Ta W Re Os Ir Pt Au Hg TI Pb Bi Po At Rn 20 1830 mabiongam 106 boh 107 ranm 87 89-102 103 104 105 108 109 110 111 112 114 Fr Ra ** Lr Rf Db Sg Bh Hs Mt Uun Uuu Uub Uuq lathanm 57 62 63 hom 67 tham 69 "70 59 60 61 64 65 66 68 *Lanthanide series La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb 14 prd 91 144 174 **Actinide series 19 97 99 90 92 93 94 96 98 100 101 102 Ac Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No p psa Select one: O a. Zn, Cd, Hg O b. Cr, Mo, W O . K, Rb, Cs O d. Sc, Y, Lu O e. Mn, Tc, Re
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter22: The Chemistry Of The Transistion Elements
Section: Chapter Questions
Problem 43PS
Related questions
Question
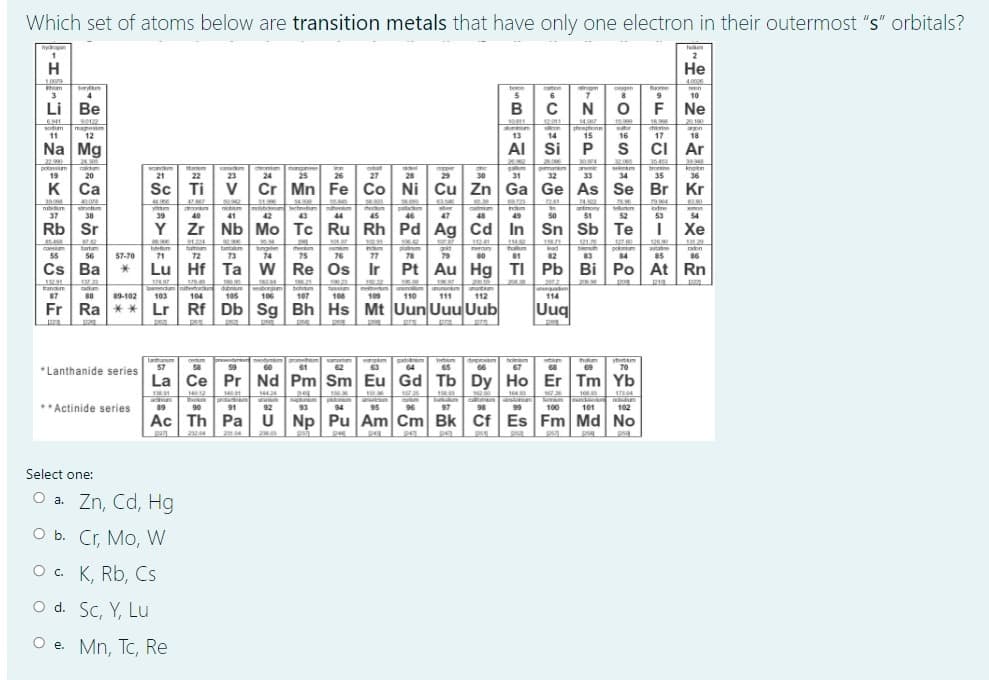
Transcribed Image Text:Which set of atoms below are transition metals that have only one electron in their outermost "s" orbitals?
1
H
Не
1.009
4.0000
m
ben
cton
ongen
3
5
6
10
4
Li Be
C
F Ne
s0122
manestm
12
14
thonphene
15
0811
12:011
16.
2010
sodm
11
13
14
16
17
18
Na Mg
AI Si
P
CI Ar
24
220
potavm
19
va m
23
mongne
25
.000
s em
34
tro
35
orm
bat
pmanm
knoon
31
28
30
Cr Mn Fe Co Ni Cu Zn Ga Ge As Se
20
21
22
24
26
27
29
32
33
36
к Са
Sc
Ti
V
Br Kr
241
mhbr h
42
pallatkm
46
242
antony
51
stnm
catmian
48
ontm
odine
37
38
39
40
41
43
44
45
47
49
50
52
53
54
Rb| Sr
Y Zr Nb Mo Tc Ru Rh Pd Ag Cd
In Sn Sb Te
Хе
sor s
hamm
72
ng
74
bemuth
83
126
astate
85
bortum
tantm
heum
meroun
tham
Iad
aon
55
56
57-70
71
73
75
76
77
78
79
80
81
82
84
86
Cs Ba
Lu Hf Ta w Re Os Ir
Pt Au Hg TI Pb Bi Po At Rn
1830
20
a don
104
bohu
107
rann
106
110
114
87
89-102
103
105
108
109
111
112
Fr Ra ** Lr Rf Db Sg Bh Hs Mt Uun Uuu Uub
Uug
ltham
57
thm
69
vt m
*Lanthanide series
I m
65
hom
67
"70
59
60
61
62
63
64
66
68
La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb
144
174
**Actinide series
19
prad
91
97
99
90
92
93
94
96
98
100
101
102
Ac Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No
psa
Select one:
O a. Zn, Cd, Hg
O b. Cr, Mo, W
O . K, Rb, Cs
O d. Sc, Y, Lu
O e. Mn, Tc, Re
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning