You decided to take the pH after each addition of 5.0 mL of NaOH rather than after every 0.5 mL during the neutralization of your acid sample. The pH buffers used to calibrate the pH meter were each 0.5 pH units high (4.5 and 7.5 rather than 4.0 and 1. pKa would be unchanged. 7.0 respectively). 2. pKa would be too high. While using the Half Volume method, your solution was dark pink (you overshot the endpoint) and added the unreacted 3. pka would be too low. you acid to this solution. You refilled your buret between titrations of your unknown acid. Instead of using 0.1 M NAOH as you did for the first titration, you used 0.2 M NaOH.
You decided to take the pH after each addition of 5.0 mL of NaOH rather than after every 0.5 mL during the neutralization of your acid sample. The pH buffers used to calibrate the pH meter were each 0.5 pH units high (4.5 and 7.5 rather than 4.0 and 1. pKa would be unchanged. 7.0 respectively). 2. pKa would be too high. While using the Half Volume method, your solution was dark pink (you overshot the endpoint) and added the unreacted 3. pka would be too low. you acid to this solution. You refilled your buret between titrations of your unknown acid. Instead of using 0.1 M NAOH as you did for the first titration, you used 0.2 M NaOH.
Chapter8: Polyfunctional Acids And Bases
Section: Chapter Questions
Problem 10P
Related questions
Question
100%
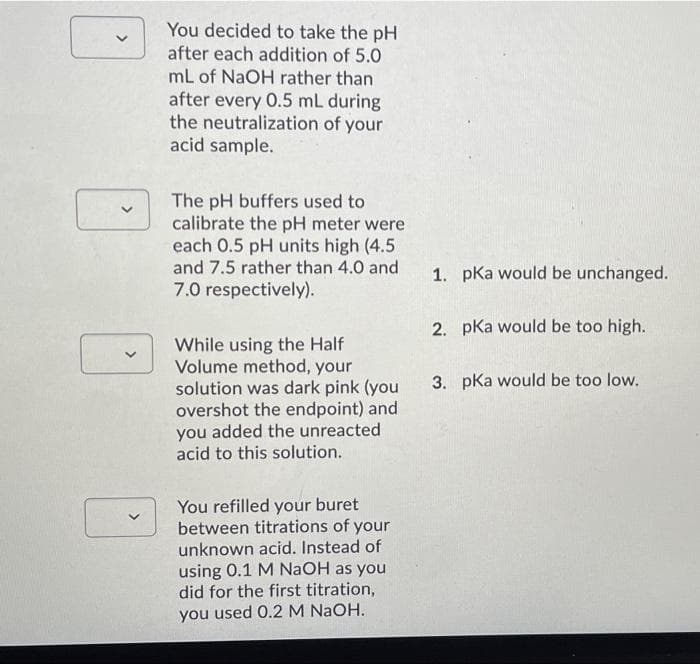
Transcribed Image Text:You decided to take the pH
after each addition of 5.0
mL of NaOH rather than
after every 0.5 mL during
the neutralization of your
acid sample.
The pH buffers used to
calibrate the pH meter were
each 0.5 pH units high (4.5
and 7.5 rather than 4.0 and
1. pka would be unchanged.
7.0 respectively).
2. pKa would be too high.
While using the Half
Volume method, your
solution was dark pink (you
overshot the endpoint) and
you added the unreacted
acid to this solution.
3. pka would be too low.
You refilled your buret
between titrations of your
unknown acid. Instead of
using 0.1 M NaOH as you
did for the first titration,
you used 0.2 M NaOH.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning



Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning
