
Concept explainers
(a)
Interpretation:
The formal charge on each atom and the net charge in the given species are to be stated.
Concept introduction:
Chemical compounds contain two types of bonds. These are known as ionic and covalent bonds. In ionic bonds, the ions are held by the electrostatic interaction between them. In covalent bonds, the atoms are held together by the sharing of electrons. The formal charge is the charge on the constituent atoms in a molecule. It is calculated by using valence electrons of the atom.
Answer to Problem 1.25AP
The formal charges on oxygen atom and chlorine atom are
Explanation of Solution
The given structure is shown in figure 1.
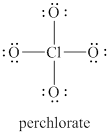
Figure 1
The formal charge on atom is calculated by the formula given below as,
The valence electrons of oxygen atoms in given species are
Thus, the formal charge on each oxygen atom is
The valence electrons of chlorine atom in given species are
Thus, the formal charge on chlorine atom is
The total charge on the given species is calculated as follows.
Hence, total charge on the species is
The formal charges on oxygen atom and chlorine atom are
(b)
Interpretation:
The formal charge on each atom and the net charge in the given species are to be stated.
Concept introduction:
Chemical compounds contain two types of bonds. These are known as ionic and covalent bonds. In ionic bonds, the ions are held by the electrostatic interaction between them. In covalent bonds, the atoms are held together by the sharing of electrons. The formal charge is the charge on the constituent atoms in a molecule. It is calculated by using valence electrons of the atom.
Answer to Problem 1.25AP
The formal charges on oxygen atom, nitrogen atom and carbon atom are
Explanation of Solution
The given structure is shown in figure 2.
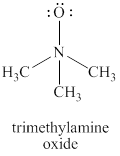
Figure 2
The formal charge on atom is calculated by the formula given below as,
The valence electrons of oxygen atoms in given species are
Thus, the formal charge on each oxygen atom is
The valence electrons of nitrogen atom in given species are
Thus, the formal charge on nitrogen atom is
The valence electrons of carbon atom in given species are
Thus, the formal charge on carbon atom is
The total charge on the given species is calculated as follows.
Hence, total charge on the species is
The formal charges on oxygen atom, nitrogen atom and carbon atom are
(c)
Interpretation:
The formal charge on each atom and the net charge in the given species are to be stated.
Concept introduction:
Chemical compounds contain two types of bonds. These are known as ionic and covalent bonds. In ionic bonds, the ions are held by the electrostatic interaction between them. In covalent bonds, the atoms are held together by the sharing of electrons. The formal charge is the charge on the constituent atoms in a molecule. It is calculated by using valence electrons of the atom.
Answer to Problem 1.25AP
The formal charges on left oxygen atom, right oxygen atom and central oxygen atom are
Explanation of Solution
The given structure is shown in figure 3.
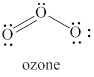
Figure 3
The formal charge on atom is calculated by the formula given below as,
The valence electrons of left oxygen atom in given species are
Thus, the formal charge on each oxygen atom is
The valence electrons of right oxygen atom in given species are
Thus, the formal charge on each oxygen atom is
The valence electrons of central oxygen atom in given species are
Thus, the formal charge on each oxygen atom is
The total charge on the given species is calculated as follows.
Hence, total charge on the species is
The formal charges on left oxygen atom, right oxygen atom and central oxygen atom are
(d)
Interpretation:
The formal charge on each atom and the net charge in the given species are to be stated.
Concept introduction:
Chemical compounds contain two types of bonds. These are known as ionic and covalent bonds. In ionic bonds, the ions are held by the electrostatic interaction between them. In covalent bonds, the atoms are held together by the sharing of electrons. The formal charge is the charge on the constituent atoms in a molecule. It is calculated by using valence electrons of the atom.
Answer to Problem 1.25AP
The formal charge on carbon atom is
Explanation of Solution
The given structure is shown in figure 4.
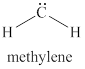
Figure 4
The formal charge on atom is calculated by the formula given below as,
The valence electrons of carbon atom in given species are
Thus, the formal charge on carbon atom is
Hence, total charge on the species is also
The formal charge on carbon atom is
(e)
Interpretation:
The formal charge on each atom and the net charge in the given species are to be stated.
Concept introduction:
Chemical compounds contain two types of bonds. These are known as ionic and covalent bonds. In ionic bonds, the ions are held by the electrostatic interaction between them. In covalent bonds, the atoms are held together by the sharing of electrons. The formal charge is the charge on the constituent atoms in a molecule. It is calculated by using valence electrons of the atom.
Answer to Problem 1.25AP
The formal charge on carbon atom and carbon radical is
Explanation of Solution
The given structure is shown in figure 5.
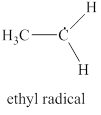
Figure 5
The formal charge on atom is calculated by the formula given below as,
The valence electrons of carbon atom in given species are
Thus, the formal charge on carbon atom is
The valence electrons of carbon radical in given species are
Thus, the formal charge on carbon radical is
Hence, total charge on the species is also
The formal charge on carbon atom and carbon radical is
(f)
Interpretation:
The formal charge on each atom and the net charge in the given species are to be stated.
Concept introduction:
Chemical compounds contain two types of bonds. These are known as ionic and covalent bonds. In ionic bonds, the ions are held by the electrostatic interaction between them. In covalent bonds, the atoms are held together by the sharing of electrons. The formal charge is the charge on the constituent atoms in a molecule. It is calculated by using valence electrons of the atom.
Answer to Problem 1.25AP
The formal charge on oxygen atom and chlorine atom is
Explanation of Solution
The given structure is shown in figure 6.
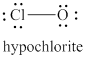
Figure 6
The formal charge on atom is calculated by the formula given below as,
The valence electrons of oxygen atoms in given species are
Thus, the formal charge on each oxygen atom is
The valence electrons of chlorine atom in given species are
Thus, the formal charge on chlorine atom is
The total charge on the given species is calculated as follows.
Hence, total charge on the species is
The formal charge on oxygen atom and chlorine atom is
Want to see more full solutions like this?
Chapter 1 Solutions
ORGANIC CHEMISTRY SAPLING ACCESS + ETEX
- The formal charge on the phosphorus atom in the resonance structures for the phosphate ion that minimizes the formal changes is ?arrow_forwardWhat is the formal charge of the carbon atom in the structure below?arrow_forwardAre the bond lengths or angles in benzene, comparedto other hydrocarbons, sufficient to decide if benzene exhibits resonanceand is especially stable? Discuss.arrow_forward
- You are given bond-line structures (which means that not all of the hydrogens are shown) and all nonzero formal charges are given. Locate and fill in all lone pair electrons in the structures.arrow_forwardDraw the best resonance structure for cyanic acid HOCN . Be sure to include all lone pair electrons and nonzero formal charges. The skeletal arrangement is given as follows.arrow_forwardCan someone please show me how the all the transfer of electrons occurs using arrows and writing out the formal charges. I'm particularly confused about the first line. Please show every single electron pair involved (even on the h2o).arrow_forward
- Write structural formulas for at least three constitutional isomers with the molecular formula ch3no2. (In answering this problem you should assign a formal charge to any atom that bears one.)arrow_forwardProvide structural(constitutional) isomers and resonance structures. Also, provide chemical formula and electron movement of resonance structures.arrow_forwardAll presented compounds contain oxygen atom in their structure. What is the formal charge on oxygen atom in each compound?arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
