
ORGANIC CHEMISTRY
4th Edition
ISBN: 9781119745105
Author: Klein
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 1, Problem 66ASP
Interpretation Introduction
Interpretation: The correct statement that described the missing formal charge(s) in the following structure should be identified:
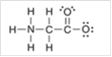
Concept Introduction: Any atom with a formal charge lacks the necessary number of valence electrons. Formal charge is assigned when an atom is present in Lewis structure.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Draw Lewis structures for tye following molecules.
a.) Atom "X" has 4 valence electrons and Atom "Y" has 6 valence electrons. Draw the Lewis structure for the molecule XY2, showing all lone pairs and formal charges when necessary
b.) Atom "M" has 5 valence electrons and Atom "L" has 7 valence electrons. Draw the Lewis structure for the molecule M2L4, showing all lone pairs and formal charges when necessary
Write a Lewis structure for each of the following molecules/ions. Be sure to show all non-zero
formal charges. Start by counting the valence electrons.
a.)Draw a Lewis diagram for IO4- in which the central I atom has a formal charge of zero and show all NONZERO formal charges on all atoms. note overall charge of ion is -1
b.)Draw a Lewis structure for IO4- in which the octet rule is satisfied on all atoms and show all NONZERO formal charges on all atoms.
C.Based on formal charge, what is the best Lewis structure for the ion? smallest formal charge or octet rule satisfied for all atoms
Chapter 1 Solutions
ORGANIC CHEMISTRY
Ch. 1.2 - Prob. 1LTSCh. 1.2 - Prob. 2ATSCh. 1.2 - Prob. 2LTSCh. 1.3 - Prob. 3LTSCh. 1.3 - Prob. 4PTSCh. 1.3 - Prob. 5PTSCh. 1.4 - Prob. 4LTSCh. 1.4 - Prob. 7PTSCh. 1.4 - Prob. 8PTSCh. 1.4 - Prob. 9ATS
Ch. 1.5 - Prob. 5LTSCh. 1.5 - Prob. 10PTSCh. 1.5 - Prob. 11ATSCh. 1.5 - Prob. 12ATSCh. 1.6 - Prob. 6LTSCh. 1.6 - Prob. 14ATSCh. 1.7 - Prob. 7LTSCh. 1.7 - Prob. 17ATSCh. 1.10 - Prob. 18CCCh. 1.10 - Prob. 20CCCh. 1.10 - Prob. 8LTSCh. 1.10 - Prob. 21PTSCh. 1.10 - Nemotin is a compound that was first isolated from...Ch. 1.10 - Prob. 23CCCh. 1.11 - Prob. 9LTSCh. 1.11 - Prob. 24PTSCh. 1.11 - Prob. 25PTSCh. 1.11 - Prob. 26PTSCh. 1.11 - Prob. 27ATSCh. 1.12 - Prob. 10LTSCh. 1.12 - Prob. 29ATSCh. 1.13 - Prob. 11LTSCh. 1.13 - Prob. 31ATSCh. 1 - Prob. 32PPCh. 1 - Prob. 33PPCh. 1 - Prob. 34PPCh. 1 - Prob. 35PPCh. 1 - Prob. 36PPCh. 1 - Prob. 37PPCh. 1 - Prob. 38PPCh. 1 - Prob. 39PPCh. 1 - Prob. 40PPCh. 1 - Prob. 41PPCh. 1 - Prob. 42PPCh. 1 - Prob. 44PPCh. 1 - Prob. 45PPCh. 1 - Prob. 46PPCh. 1 - Prob. 47PPCh. 1 - Prob. 48PPCh. 1 - Prob. 49PPCh. 1 - Prob. 50PPCh. 1 - Prob. 51PPCh. 1 - Prob. 52PPCh. 1 - Prob. 53PPCh. 1 - Prob. 54PPCh. 1 - Nicotine is an addictive substance found in...Ch. 1 - Prob. 56PPCh. 1 - Prob. 57PPCh. 1 - Prob. 59PPCh. 1 - Prob. 63ASPCh. 1 - Prob. 64ASPCh. 1 - Prob. 66ASPCh. 1 - Prob. 69ASPCh. 1 - Prob. 71ASPCh. 1 - Prob. 72ASPCh. 1 - Prob. 75IP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Which of the following is the correct guideline in choosing plausible Lewis structure? a. Lewis structure with large formal charges is preferable than with small formal charges b. The negative formal charge is placed on the more electronegative atom c. Lewis structure with many formal charges is preferable d. Lewis structure without formal charge is less plausible than with formal chargesarrow_forwardChoose the INCORRECT statement. In a Lewis structure, the number of valence electrons shown is one more for each negative charge. The central atom is typically the atom with the highest electronegativity. Formal charges are apparent charges associated with atoms in a Lewis structure. Resonance is when more than one plausible structure can be written but the "correct" structure cannot be written.arrow_forwardDraw Lewis structure(s) for the acetate ion (CH2CO0). If there are equivalent resonance structures, draw all of them. • Draw one structure per sketcher box, and separate added sketcher boxes with the Do not include overall ion charges or formal charges in your drawing. • Do not draw double bonds to oxygen unless they are needed in order for the central atom to obey the octet rule. symbol. CH3COO":arrow_forward
- Steps for Lewis Structures: 1. Determine the total number of valence electrons. Add electrons for negative charges, subtract electrons for positive charges. 2. Place least electronegative atom (except H) as central atom in structure. 3. Connect atoms by singles bonds. Each single bond = 2 electrons. 4. “Sprinkle” remaining electrons around outside atoms first to complete octets. Don’t use more electrons than the total found in step 1. Then complete the central atom’s octet last if you have enough electrons. 5. Make double or triple bonds as needed to complete octets. 6. Place brackets and charge for ions. For the central atom in each formula, draw the Lewis Structure with all valence electrons shown. 1. PH3 2. H2O 3. CO2 4. CHCl3 5. O2 6. N2 7. CF4 8. C3H8 9. CH3COOH 10. N2O 11. OCN-arrow_forwardDetermine if the structural formula below is an acceptable Lewis structures for organic compounds. Point out the problems in cases where structure is invalid. •. :OH CH- .CH3 A. Non of the options here are correct. B. This is not a correct Lewis structural formula because carbon cannot have five bonds. C. This structure is a correct Lewis structural formula. D. This is not a correct Lewis structure because the methyl group does not have an octet of electrons.arrow_forwardConsider the incomplete structure shown. Draw an alternative Lewis (resonance) structure for the incomplete structure. Show the unshared electron pairs and nonzero formal charges in your structure. Don't use radicals. Determine the formal charge on the nitrogen atom in the structure. If the atom is formally neutral, indicate a charge of zero.arrow_forward
- 1. Consider serine (it is expected you will need to refer to a chart of the 20 common amino acids to find structure information for this and other amino acids). a. Draw its complete Lewis structure of serine (show all atoms, bonds and lone pairs). Draw the version of the structure without any charges b. Identify all of the functional groups C. Draw the Zwitterion form of serine d. Explain how the Zwitterion is formed from the uncharged versionarrow_forwardTwo major resonance structures are possible for the anion shown. One resonance form is given, but it is incomplete. Complete the given structure by adding nonbonding electrons and formal charges. Draw the remaining structure, including nonbonding electrons and formal charges. Omit curved arrows. Structure A: complete the structure by adding nonbonding electrons and formal charges. H H H I Structure B: draw the remaining resonance structure, including nonbonding electrons and formal charges. H- : z: H Harrow_forward2. In biologically relevant molecules, carbon and the halogens don't have a charge. In contrast, nitrogen (neutral or positively charged) and oxygen (neutral or negatively charged) don't always appear in their uncharged form. Consider the following questions. a. Add any unshared pairs of electrons necessary to complete the Lewis structure shown for formaldehyde (note: there are no formal charges in this molecule). b. Assign any formal charges necessary to complete the structure shown for the acetylide anion. c. Recalling that atoms want a complete octet, add unshared pairs of electrons, and assign formal charges as appropriate to the Lewis structure of the amino acid aspartic acid as it would exist at a physiological pH of ~7.4 (aspartic acid has an overall -1 charge at this pH). A. H O H formaldehyde B. H C C: C. H H \/ acetylide anion H-N O=C H он O H aspartic acidarrow_forward
- Counting available electrons and drawing a Lewis structuresarrow_forwardWrite all the possible resonance structure(s). Show the movement of electrons by drawing arrow(s). Indicate the formal charges and identify the most stable structure for the compound. ö: а. b. a.arrow_forwardAdding electrons to the skeleton by making single bonds between all bonded atoms gives Each hydrogen atom now has a pair of electrons, but each carbon has only 6 electrons. Adding a pair of electrons to each carbon gives the trial structure The number of electrons in the trial structure is ____. Since this number exceeds the number of available valence electrons, the structure is incorrect.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning

 Chemical Principles in the LaboratoryChemistryISBN:9781305264434Author:Emil Slowinski, Wayne C. Wolsey, Robert RossiPublisher:Brooks Cole
Chemical Principles in the LaboratoryChemistryISBN:9781305264434Author:Emil Slowinski, Wayne C. Wolsey, Robert RossiPublisher:Brooks Cole Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning



Chemical Principles in the Laboratory
Chemistry
ISBN:9781305264434
Author:Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:Brooks Cole

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co
Chapter 4 Alkanes and Cycloalkanes Lesson 2; Author: Linda Hanson;https://www.youtube.com/watch?v=AL_CM_Btef4;License: Standard YouTube License, CC-BY
Chapter 4 Alkanes and Cycloalkanes Lesson 1; Author: Linda Hanson;https://www.youtube.com/watch?v=PPIa6EHJMJw;License: Standard Youtube License