
Concept explainers
(a)
Interpretation:
The empirical formula of the given hydrocarbon has to be determined.
Concept Introduction:
The hydrocarbon compounds are compounds which contains only carbon and hydrogen atoms. The combustion of hydrocarbon compounds produces large amount of heat. Combustion reaction of a hydrocarbon results in the formation of carbon dioxide and water.
(a)
Answer to Problem 101QRT
The empirical formula of given hydrocarbon is
Explanation of Solution
The mass of carbon dioxide produced is
The mass of water produced is
The molar mass of carbon dioxide is
The molar mass of water is
Use the expression to calculate number of moles.
Substitute
Therefore, the number of moles of carbon dioxide is
Consider the combustion reaction of hydrocarbon as follows.
Here,
When combustion of hydrocarbon takes place, the carbon atoms present in hydrocarbon gets converted to carbon dioxide molecule. One molecule of carbon dioxide contains one carbon atom. Therefore, the number of moles of carbon atoms present in hydrocarbon is equal to the moles of carbon dioxide.
Therefore, moles of carbon atom in hydrocarbon is
Substitute
Therefore, number of moles of water is
When the combustion of hydrocarbon takes place the hydrogen atoms present in hydrocarbon gets converted into hydrogen atoms of water molecule. One molecule of water contains two hydrogen atoms. Therefore, the number of moles of hydrogen atom present in hydrocarbon is twice the number of moles of water.
Therefore, the number of moles of hydrogen atoms is
The smallest number of moles is
Use the expression to calculate mole ratio.
Substitute
Substitute
Therefore, the empirical formula of given hydrocarbon is
(b)
Interpretation:
The given hydrocarbon is an
Concept Introduction:
Refer to part (a).
(b)
Answer to Problem 101QRT
The given hydrocarbon compound is an alkene.
Explanation of Solution
The general formula of alkane is
(c)
Interpretation:
The Lewis structure for given hydrocarbon has to be drawn.
Concept Introduction:
Refer to part (a).
(c)
Answer to Problem 101QRT
The Lewis structure for given hydrocarbon is as follows.
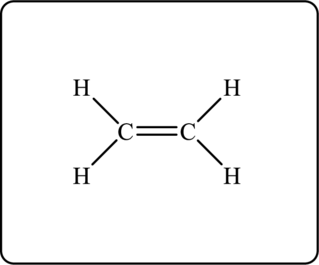
Another possible structure is as follows.
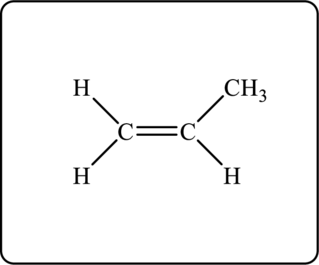
Explanation of Solution
The given hydrocarbon has empirical formula
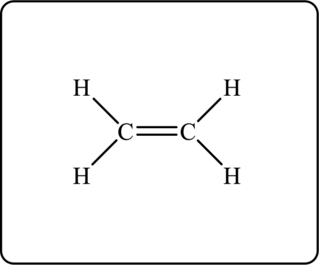
Figure 1
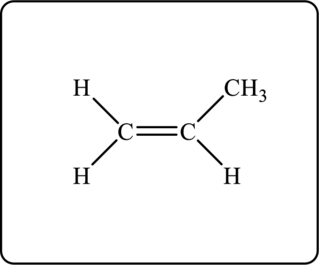
Figure 2
Want to see more full solutions like this?
Chapter 10 Solutions
Chemistry: The Molecular Science
- Is cis,trans isomerism possible in alkanes?arrow_forwardGive the molecular formula of a hydrocarbon containing five carbon atoms that is an alkane.arrow_forwardGive the molecular formula of a hydrocarbon containingfive carbon atoms that is (a) an alkane, (b) a cycloalkane,(c) an alkene, (d) an alkyne.arrow_forward
- 1. Write equations for the substitution of hydrogen by bromine in methane.2. Write the reaction for the combustion of ethane.arrow_forwardDraw all the structural and geometric isomers of pentene, C5H10, that have an unbranched hydrocarbon chain.arrow_forwardwhat are the functional groups in the molecule C3H8O?arrow_forward
- write the structure formulas of alkanes with molecular formula C6H14, which with chlorine give: a) three monochlorinated isomers? b) five monochlorinated isomers c) only two monochlorinated isomersarrow_forwardDraw the structure and give the molecular formula for a compound(a) 3-ethyl-2,4-dimethylhexanearrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning


