
Concept explainers
(a)
Interpretation:
The molecular formula of the hydrocarbon has to be derived.
Concept Introduction:
The compounds that contain carbon and hydrogen atoms are known as hydrocarbon compounds. There are two classes of hydrocarbon compounds which are saturated and
Saturated hydrocarbon compound has only one type that is
(a)
Explanation of Solution
The number of moles of hydrocarbon is calculated by the formula shown below.
The mass of hydrocarbon is
The molar mass of hydrocarbon is
Substitute the values in the above formula.
The volume of
Thus, the number of moles of hydrogen occupied by
The moles of hydrogen per moles of hydrocarbon are calculated as shown below.
Thus, one mole of hydrocarbon reacts with two moles of hydrogen. This indicates the presence of two double or one triple bond in the hydrocarbon.
The mass percent of carbon is
The mass percent of hydrogen is
The mass of carbon is calculated as shown below.
The mass of hydrogen is calculated as shown below.
The number of moles of carbon is calculated by the formula shown below.
The mass of carbon is
The molar mass of carbon is
Substitute the values in the above formula.
The number of moles of hydrogen is calculated by the formula shown below.
The mass of hydrogen is
The molar mass of hydrogen n is
Substitute the values in the above formula.
The molar ratio of
Thus, the molecular formula of the hydrocarbon is
(b)
Interpretation:
The structural formula for two possible isomers has to be drawn.
Concept Introduction:
The molecules which have same molecular formula and same connectivity of atoms but different arrangement of atoms in space are known as stereoisomers. Stereocentre is the region or atom in a molecule due to which molecule is showing stereoisomerism. The interchange of groups at stereocentre generates stereoisomer.
(b)
Explanation of Solution
The molecular formula of the hydrocarbon is
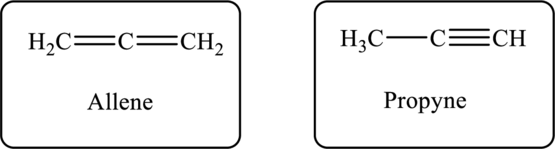
Figure 1
(c)
Interpretation:
The balanced chemical equation has to be used to show the bromination and hydrogenation reaction for one of the isomers.
Concept Introduction:
The addition of a bromine atom in the given compound is known as bromination. Bromination occurs through an electrophilic substitution reaction. Bromine atom acts as an electrophile which causes the formation of sigma bond in the reaction.
(c)
Explanation of Solution
Propyne undergoes bromination to form tetrabromo product. The balanced chemical equation is shown below.
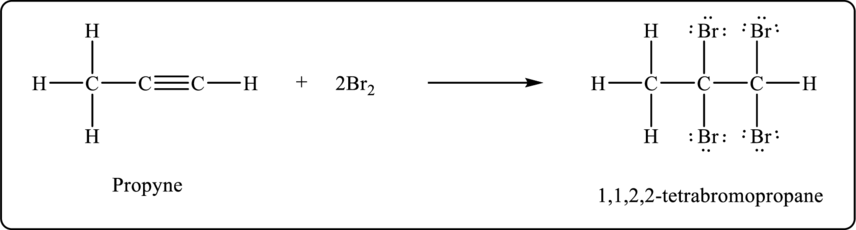
Figure 2
Propyne undergoes hydrogenation to form propane. The balanced chemical equation is shown below.
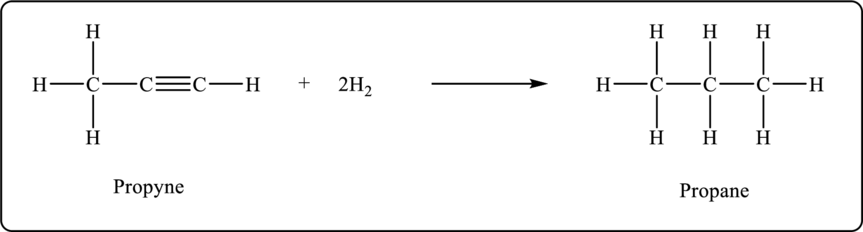
Figure 3
Want to see more full solutions like this?
Chapter 10 Solutions
Chemistry: The Molecular Science
- Write a balanced chemical reaction for the incomplete combustion of pentane, C5H12C5H12 in oxygen gas to form carbon monoxide gas as one of the products.arrow_forwardwrite the structure formulas of alkanes with molecular formula C6H14, which with chlorine give: a) three monochlorinated isomers? b) five monochlorinated isomers c) only two monochlorinated isomersarrow_forwardDescribe structural isomerism and the different types that exist. With the aid of diagrams use the molecular formulae C5H12 and C5H10 to explain structural isomerism in aliphatic alkanes and alkenesarrow_forward
- Enter the molecular formula for butane, C4H10?arrow_forwardFor the four classes of hydrocarbons (Alkane, Alkene, Alkyne, Arene): Give a structural formula example of each, or identify the characteristic bond that is present.arrow_forward1. Write equations for the substitution of hydrogen by bromine in methane.2. Write the reaction for the combustion of ethane.arrow_forward
- compare and contrats the physical and chemical properties of open-chain versus closed-chain hydrocarbonsarrow_forwardWrite the polymerization reaction in which acetylene (C2H2) produces polyacetylene. Show the structural formulae of the monomers and the polymer.arrow_forwardGive the molecular formula of a hydrocarbon containingfive carbon atoms that is (a) an alkane, (b) a cycloalkane,(c) an alkene, (d) an alkyne.arrow_forward
- identify some common functional groups of organicmolecules from their formulaearrow_forwardWrite the structural formula of 2-ethyl-1-butenearrow_forward(a) When the metallic element sodium combines with the nonmetallic element bromine, Br2(l), how can you determine the chemical formula of the product? How do you know whether the product is a solid, liquid, or gas at room temperature? Write the balanced chemical equation for the reaction. (b) When a hydrocarbon burns in air, what reactant besides the hydrocarbon is involved in the reaction? What products are formed? Write a balanced chemical equation for the combustion of benzene C6H6(l), in air.arrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning



