
Concept explainers
Answer the questions in Problem 10.35 for the
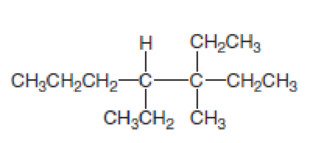
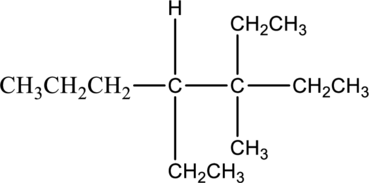
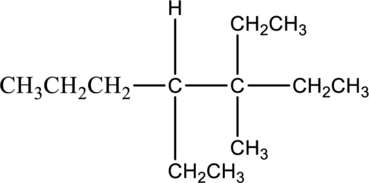
Answer the following questions about the alkane drawn below.
- a. Give the IUPAC name.
- b. Draw one constitutional isomer.
- c. Predict the solubility in water.
- d. Predict the solubility in an organic solvent.
- e. Write a balanced equation for complete combustion.
a.
Interpretation:
The IUPAC name of given compound has to be identified.
Concept Introduction:
IUPAC Nomenclature:
The IUPAC Nomenclature is a systematic writing name for organic compounds. The full form of IUPAC is known as International Union of Pure and Applied Chemistry.
There are some rules followed for writing IUPAC Nomenclature for alkane compound is given below,
- The longest chain in the compound is known as parent name which represent the number of carbon atom present in the continuous carbon chain. The root name for alkane compound is –ane. The single bonds are formed in alkane compounds.
- The suffix group denotes the functional group present in a molecule. The prefix group indicates the identity, location and number of substituents attached to the carbon compound.
- Then name and number the substituents present in compounds. Then use prefix di-to represent two groups, tri- refers to three groups and so on.
- Then numbers has to be separated by using commas and the letters from numbers has to be separated by using dashes.
Explanation of Solution
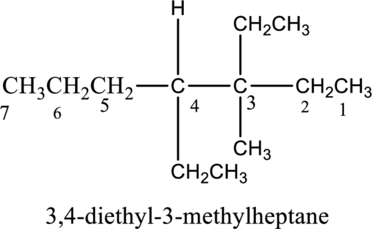
In this compound, the longest continuous chain has seven carbon atoms; so the parent name can be given as hept- and add suffix name –ane for alkane molecule. The parent name of given compound is heptane. Then number the substituents in alphabetical order. The ethyl group
The IUPAC name of compound is given below,
b.
Interpretation
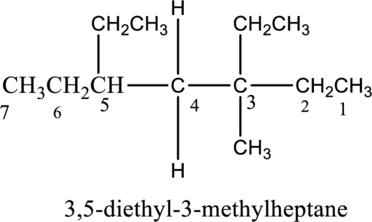
The constitutional isomer of 3, 4-diethyl-3-methylheptane has to be explained.
Concept Introduction:
Isomers:
Isomers contain two or more compounds in which the molecular formula of compounds is same but the atoms are arranged in different order. The arrangement of atoms will be in different manner.
Constitutional isomer:
The compounds have the same molecular formula but atoms are arranged in different connectivity is known as constitutional isomer.
Explanation of Solution
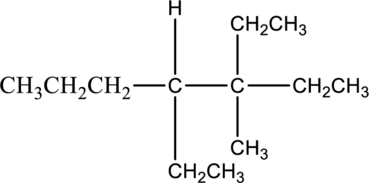
The isomer 3,4-diethyl-3-methylheptane has seven carbon atoms present in the longest chain. The two substituents ethyl groups
The constitutional isomers of 3,4-diethyl-3-methylheptane is given below,
c.
Interpretation:
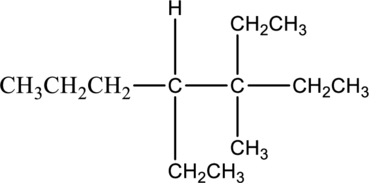
The solubility of given compound in water has to be predicted.
Concept Introduction:
Solubility:
The solubility is defined as the solid or liquid or gaseous substance which dissolves in suitable solvent. The nonpolar molecule has no separate positive and negative charge. Polar molecules have separate positive and negative charges.
Explanation of Solution
The compound 3,4-diethyl-3-methylheptane is insoluble in water. The water molecule is said to be polar molecules. It has both positive and negative charge. The given compound is a nonpolar alkane. The nonpolar alkanes cannot soluble in water molecule. Hence the given compound 3,4-diethyl-3-methylheptane is insoluble in water.
d.
Interpretation:
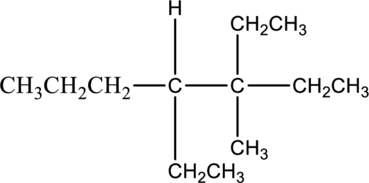
The solubility of given compound in organic compound has to be predicted.
Concept Introduction:
Solubility:
The solubility is defined as the solid or liquid or gaseous substance which dissolves in suitable solvent. The nonpolar molecule has no separate positive and negative charge. The Polar molecules have separate positive and negative charges.
Explanation of Solution
The given compound 3,4-diethyl-3-methylheptane is soluble in organic solvent. The reason for the solubility of the given compound, it belongs to nonpolar molecule. Nonpolar molecules cannot soluble in polar molecule. The nonpolar molecule has no separate charges. Hence given molecule 3,4-diethyl-3-methylheptane is soluble in organic solvent.
e.
Interpretation:
The balanced equation for complete combustion of given compound has to be written.
Concept Introduction:
Combustion reaction:
The hydrocarbon compound reacts with oxygen to form carbon dioxide and water as product is known as hydrocarbon combustion reaction. It is an exothermic reaction. The hydrocarbon compounds are generally organic compounds contain carbon and hydrogen element. The compounds such as alkanes, alkenes, alkynes and aromatic compounds.
The general equation is given as,
Where,
- x represents the number of carbon atoms present in the hydrocarbon
- y represents the number of carbon atoms present in the hydrocarbon
- N represents the number of oxygen atoms requires in the hydrocarbon combustion reaction
Chemical equation:
In chemical equation, the substance reacts (reactant) to give products. The number of atoms present in reactant side should be equal to the number of atom present in the product side.
Explanation of Solution
The compound 3,4-diethyl-3-methylheptane
The chemical reaction for complete combustion of ethane is given below,
In reactant side, it has twenty four carbon atoms and fifty two hydrogen and seventy four atoms oxygen atoms is present. In product side there are twenty four carbon atoms and forty eight oxygen atoms present in carbon dioxide. The fifty six hydrogen atoms and twenty six oxygen atoms are present in water molecule. In this reaction the molecule
The balanced equation for complete combustion reaction of 3-ethyl-4,4-dimethylheptane is given below,
Want to see more full solutions like this?
Chapter 10 Solutions
General, Organic, & Biological Chemistry
- Draw structural formulas and give IUPAC names for all the isomeric pentenes (C5H10) that are: a.Alkenes that do not show geometric isomerism. There are four compounds. b.Alkenes that do show geometric isomerism. There is one cis and one trans compound.arrow_forwardCompare the reactivity of alkanes and Alkenes.arrow_forwardQuestion 10: Draw the structure of the alkene that will give the alcohol in Figure 10 as the main product. [Give the answer in SMILES notation. Draw the structure in the editor at https://jsme-editor.github.io/dist/JSME_test.html, generate the SMILES notation, copy it and paste it as the answer to this question.] *arrow_forward
- 1. What is the molecular formula for the alkane shown in the model? Express your answer as a chemical formula. 2. Name the alkane shown in the model. Express your answer as the IUPAC name.arrow_forwardDraw the structure of any secondary alkyl halide and give the IUPAC name of the compoundarrow_forwardDraw the structure of 3-benzylpentane.arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning





