
Concept explainers
(a)
Interpretation:
Possible number of stereoisomers for the given compound has to be detected.
Concept Introduction:
Stereoisomers: The molecule that have same molecular formula and sequence of bonded atoms, but different in the three-dimensional orientation of their in space.
Chiral: Chiral molecules contain at least one carbon atom with four non-identical substitutions (or) groups. This type of molecule is called a chiral center.
Diastereomers: This type of stereoisomers that ae not mirror images of one another and non-superimposable on one another. Molecule with two or more stereocenters it can be diastereomers.
Meso isomers: The molecule with multiple stereocenters that is superimposable on tit mirror images. Meso compounds are achiral that are multiple chiral center.
(b)
Interpretation:
The stereoisomers of given compound that can be formed from oxidation of (S)-4-methylcyclohexene with Osmium tetroxide has to be predicted.
Concept Introduction:
Stereoisomers: The molecule that have same molecular formula and sequence of bonded atoms, but different in the three-dimensional orientation of their in space.
An
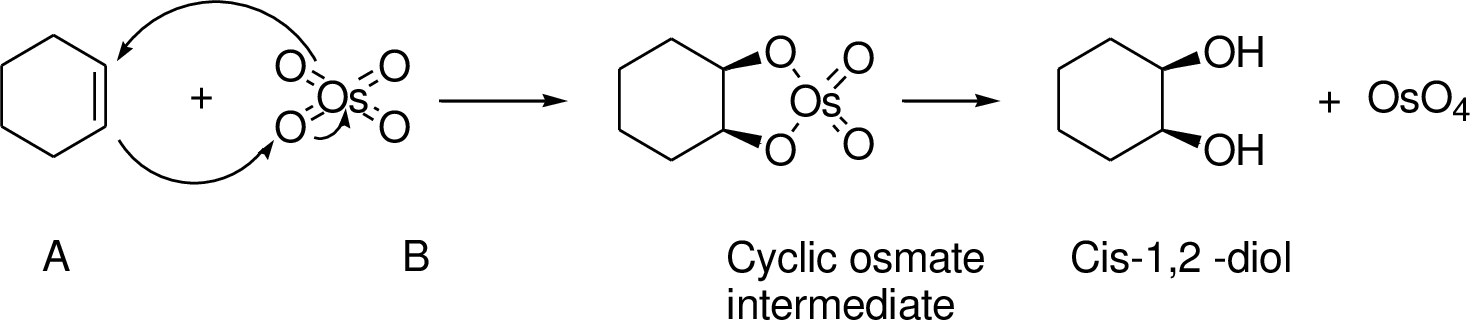
(c)
Interpretation:
Whether product formed in part (b) is either optically active or not has to be predicted.
Concept Introduction:
Stereoisomers: The molecule that have same molecular formula and sequence of bonded atoms, but different in the three-dimensional orientation of their in space.
Chirality: The presence of four different atoms (or groups) at carbon is known as asymmetric carbon and is known as chiral center of the compound. Chiral compounds are optically active.
Trending nowThis is a popular solution!

Chapter 10 Solutions
Organic Chemistry
- Which of the isomeric alcohols having the molecular formula C6H14O are chiral? Which are achiral?arrow_forward(a) Why does p-dichlorobenzene have a higher m.p. than its o- and m-isomers?(b) Why is (±)-Butan-2-ol optically inactive?arrow_forwardKetones react with alcohols to yield products called acetals. Why does the all-cis isomer of 4-tert-butyl-1,3-cyclohexanediol react readily with acetone and an acid catalyst to form an acetal, but other stereoisomers do not react? In formulating your answer, draw the more stable chair conformations of all four stereoisomers and the product acetal for each one.arrow_forward
- Five isomeric alkanes (A–E) having the molecular formula C6H14 are each treated with Cl2 + hv to give alkyl halides having molecular formulaC6H13Cl. A yields five constitutional isomers. B yields four constitutionalisomers. C yields two constitutional isomers. D yields threeconstitutional isomers, two of which possess stereogenic centers. Eyields three constitutional isomers, only one of which possesses astereogenic center. Identify the structures of A–E.arrow_forwardThe bicyclic alkene P can be prepared by thermal electrocyclic ring closure from cyclodecadiene Q or by photochemical electrocyclic ring closure from cyclodecadiene R. Draw the structures of Q and R, and indicate the stereochemistry of the process by which each reaction occurs.arrow_forward2-Acetoxycyclohexyl tosylate reacts with acetate ion to form 1,2-cyclohexanediol diacetate. The reaction is stereospecific—that is, the stereoisomers obtained as products depend on the stereoisomer used as a reactant. Recall that because 2-acetoxycyclohexyl tosylate has two asymmetric centers, it has four stereoisomers—two are cis and two are trans. Explain the following observations:a. Both cis reactants form an optically active trans product, but each cis reactant forms a different trans product.b. Both trans reactants form the same racemic mixture.c. A trans reactant is more reactive than a cis reactant.arrow_forward
- 2-Acetoxycyclohexyl tosylate reacts with acetate ion to form 1,2-cyclohexanediol diacetate. The reaction is stereospecific—that is, the stereoisomers obtained as products depend on the stereoisomer used as a reactant. Recall that because 2-acetoxycyclohexyl tosylate has two asymmetric centers, it has four stereoisomers—two are cis and two are trans. Explain the following observations: a. Both cis reactants form an optically active trans product, but each cis reactant forms a different trans product. b. Both trans reactants form the same racemic mixture. c. A trans reactant is more reactive than a cis reactantarrow_forwardThe two stereoisomers of cinnamic acid (C6H5CH=CHCO2H) each give a different product on reaction with 1,3-butadiene. Write an equation for each reaction.arrow_forwardAs we learned, monosubstituted cyclohexanes exist as anequilibrium mixture of two conformations having either an axial orequatorial substituent. When R = CH2CH3, Keq for this process is 23.When R = C(CH3)3, Keq for this process is 4000. Question: a.) Which R shows the higher percentage of axial conformation at equilibrium?arrow_forward
- Write the structure of the compound E,E-2,4-Hexadien-1-ol and label each non-equivalent carbon with a letter, A,B,C..arrow_forwardAccount for the regioselectivity and stereoselectivity observed when 1-methylcyclopentene is treated with reagent. Q) Br2 in H2Oarrow_forwardWhat is the systematic name for the 1,2,4-trichlorocyclohexane stereoisomer with the smallest heat of combustion?arrow_forward
