
(a)
Interpretation: The products for the given reaction including stereoisomers are to be drawn.
Concept introduction: Hydration of
The general steps involved in the hydration reaction are stated below:
• First, protonation of the alkene takes place to generate the carbocation.
• Formation of protonated alcohol.
• Deprotonation.
In this type of reaction, hydroxyl group attacks on the more substituted carbon.
Stereochemistry is defined as the arrangement of molecules in three dimensional space and its impact on
Answer to Problem 10.57P
The products for the given reaction including stereoisomers are shown below.
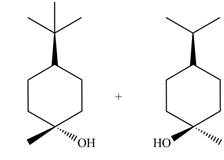
Explanation of Solution
Hydration of alkenes is one of the methods used for the formation of alcohol.
The general steps involved in the hydration reaction are stated below:
• First, protonation of the alkene takes place to generate the carbocation.
• Formation of protonated alcohol.
• Deprotonation.
In this type of reaction, hydroxyl group attacks on the more substituted carbon.
The given reaction is,
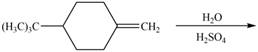
Figure 1
The products for the given reaction including stereoisomers are shown below.
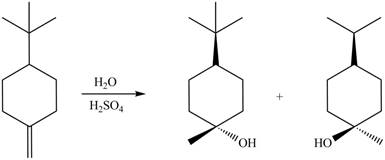
Figure 2
The products for the given reaction including stereoisomers are shown in Figure 2.
(b)
Interpretation: The products for the given reaction including stereoisomers are to be drawn.
Concept introduction: The reaction of hydrogen halide with alkene results in the formation of
Electrophilic addition reactions are those in which breaking of pi bond takes place to form new sigma bond. In this type of reaction, carbocation is formed during the formation of new bond.
Answer to Problem 10.57P
The products for the given reaction including stereoisomers are shown below.
Explanation of Solution
Electrophilic addition reaction follows Markovnikov’s rule. According to Markovnikov’s rule, the positive part of halogen acid attached to that carbon atom in
The steps involved in the electrophilic addition reaction are stated below:
• First, protonation of the alkene takes place to generate the carbocation.
• The halide ion will attack on the carbocation to give the final product.
The given reaction is,
![]()
Figure 3
The products for the given reaction including stereoisomers are shown below.
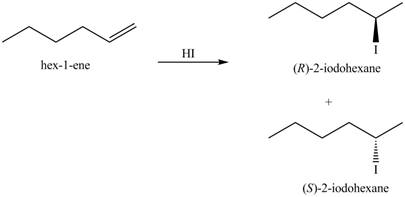
Figure 4
The products for the given reaction including stereoisomers are shown in Figure 4.
(c)
Interpretation: The products for the given reaction including stereoisomers are to be drawn.
Concept introduction: The addition of Halogens to alkenes is a stereospecific reaction. The resultant product is meso compound, if alkene is cis and addition of halogen is syn addition. If addition of halogen is anti, then the resultant products are racemic mixture. In case of trans alkene, the resultant product is meso, if addition of halogen is anti. The resultant products are racemic mixture, if the addition of halogen is syn.
Answer to Problem 10.57P
The products for the given reaction including stereoisomers are shown below.
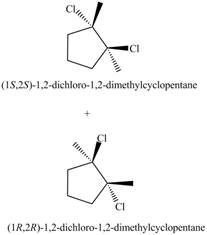
Explanation of Solution
The general steps involved in the addition of Halogens to alkenes are stated below:
• The first step is the electrophilic attack of halide ion to form a halonium ion.
• The second step is the attack of halide ion from back side to open the halonium ion.
The given reaction is,
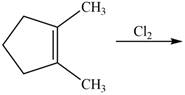
Figure 5
The products for the given reaction including stereoisomers are shown below.
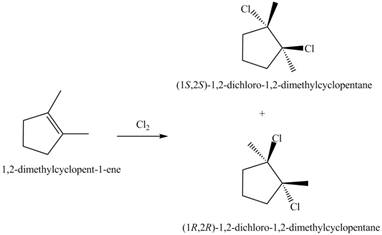
Figure 6
The products for the given reaction including stereoisomers are shown in Figure 6.
(d)
Interpretation: The products for the given reaction including stereoisomers are to be drawn.
Concept introduction: Hydroboration reaction is a two step reaction, which involves conversion of alkene into alcohol. This type of reaction follows anti-Markovnikov’s rule.
Anti markovnikov rule states that the positive part of acid gets attached to that carbon atom in
Anti addition is the addition of any atom from the opposite faces of the double bond of alkene, whereas syn addition is the addition of atom from the same side.
Answer to Problem 10.57P
The products for the given reaction including stereoisomers are given below.
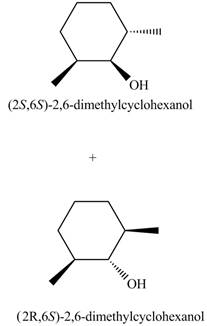
Explanation of Solution
During hydroboration reaction, if boron and hydrogen adds to the double bond of alkene from the same side then it leads to formation of syn addition, whereas if they add from the opposite side then it leads to anti-addition.
The given reaction is,
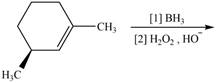
Figure 7
The products for the given reaction including stereoisomers are shown below.
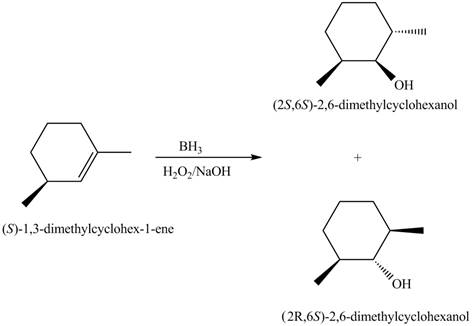
Figure 8
The products for the given reaction including stereoisomers are shown in Figure 8.
(e)
Interpretation: The products for the given reaction including stereoisomers are to be drawn.
Concept introduction: The reaction of alkene with halogen and water results in the formation of halohydrin product.
Stereochemistry is defined as the arrangement of molecules in three dimensional space and its impact on chemical reactions. Anti stereochemistry is the addition of any atom from the opposite faces of the double bond of alkene, whereas syn addition is the addition of atom from the same side.
The general steps for the formation of halohydrin are stated below:
• The first step is the attack of halide ion to form a halonium ion.
• The second step is the attack of water from back side to open the halonium ion.
• The last step is deprotonation to give the halohydrin product.
Answer to Problem 10.57P
The product for the given reaction including stereoisomers is given below.
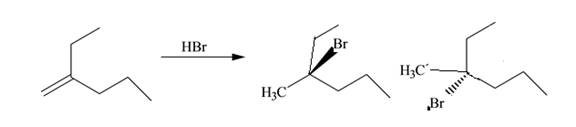
Explanation of Solution
The addition of halogens to alkenes is a stereospecific reaction. The resultant product is meso compound, if alkene is cis and addition of halogen is syn addition. If addition of halogen is anti, then the resultant products are racemic mixture. In case of trans alkene, the resultant product is meso, if addition of halogen is anti. The resultant products are racemic mixture, if the addition of halogen is syn addition.
The given reaction is,
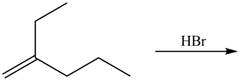
Figure 9
The general steps for the formation of halohydrin are stated below:
• The first step is the attack of halide ion to form a halonium ion.
• The second step is the attack of water from back side to open the halonium ion.
• The last step is deprotonation to give the halohydrin product.
The products for the given reaction including stereoisomers are shown below.
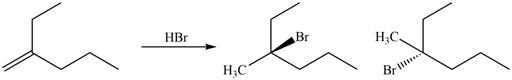
Figure 10
The products for the given reaction including stereoisomers are shown in Figure 10.
(f)
Interpretation: The products for the given reaction including stereoisomers are to be drawn.
Concept introduction: The reaction of alkene with halogen and water results in the formation of halohydrin product.
Stereochemistry is defined as the arrangement of molecule in three dimensional and its impact on chemical reactions. Anti stereochemistry is the addition of any atom from the opposite faces of the double bond of alkene, whereas syn addition is the addition of atom from the same side.
The general steps for the formation of halohydrin are stated below:
• The first step is the attack of halide ion to form a halonium ion.
• The second step is the attack of water from back side to open the halonium ion.
• The last step is deprotonation to give the halohydrin product.
Answer to Problem 10.57P
The product for the given reaction including stereoisomers is given below.
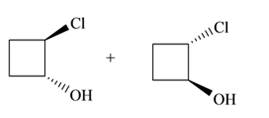
Explanation of Solution
The addition of halogens to alkenes is a stereospecific reaction. The resultant product is meso compound, if alkene is cis and addition of halogen is syn addition. If addition of halogen is anti, then the resultant products are racemic mixture. In case of trans alkene, the resultant product is meso, if addition of halogen is anti. The resultant products are racemic mixture, if the addition of halogen is syn addition.
The given reaction is,
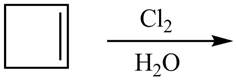
Figure 11
The general steps for the formation of halohydrin are stated below:
• The first step is the attack of halide ion to form a halonium ion.
• The second step is the attack of water from back side to open the halonium ion.
• The last step is deprotonation to give the halohydrin product.
The products for the given reaction including stereoisomers are shown below.
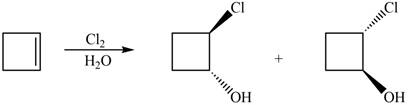
Figure 12
The products for the given reaction including stereoisomers are shown in figure 12.
(g)
Interpretation: The products for the given reaction including stereoisomers are to be drawn.
Concept introduction: Hydration of alkenes is one of the methods used for the formation of alcohol.
The general steps followed by hydration reaction are stated below:
• First, protonation of the alkene takes place to generate the carbocation.
• Formation of protonated alcohol.
• Deprotonation.
In this type of reaction, hydroxyl group attacks on the more substituted carbon.
Stereochemistry is defined as the arrangement of molecule in three dimensional and its impact on chemical reactions. Stereochemistry for the products is shown by the dashed and wedge lines. Dashed lines represent that the bond is far away from the plane, whereas wedge line represents the bond out of the plane and towards the observer. These lines are used to represent the stereochemistry of the compound.
Answer to Problem 10.57P
The products for the given reaction including stereoisomers are shown below.
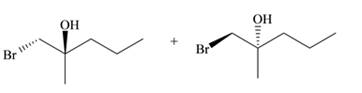
Explanation of Solution
The addition of Halogens to alkenes is a stereospecific reaction. The resultant product is meso compound, if alkene is cis and addition of halogen is syn addition. If addition of halogen is anti, then the resultant products are racemic mixture. In case of trans alkene, the resultant product is meso, if addition of halogen is anti. The resultant products are racemic mixture, if the addition of halogen is syn addition.
The given reaction is,
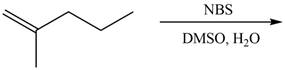
Figure 13
The reaction of
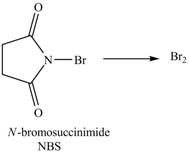
Figure 14
The general steps for the formation of halohydrin are stated below:
• The first step is the attack of halide ion to form a halonium ion.
• The second step is the attack of water from back side to open the halonium ion.
• The last step is deprotonation to give the halohydrin product.
The products for the given reaction including stereoisomers are shown below.
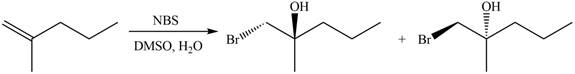
Figure 15
The attack of water on halonium ion is from back side that opens the bromonium ion to form mixture of enantiomers. This shows that the mode of addition in this reaction is anti which gives trans enantiomers.
The products for the given reaction including stereoisomers are shown in figure 15.
(h)
Interpretation: The products for the given reaction including stereoisomers are to be drawn.
Concept introduction: Hydration of alkenes is one of the methods used for the formation of alcohol.
The general steps followed by hydration reaction are stated below:
• First, protonation of the alkene takes place to generate the carbocation.
• Formation of protonated alcohol.
• Deprotonation.
In this type of reaction, hydroxyl group attacks on the more substituted carbon.
Stereochemistry is defined as the arrangement of molecule in three dimensional and its impact on chemical reactions. Stereochemistry for the products is shown by the dashed and wedge lines. Dashed lines represent that the bond is far away from the plane, whereas wedge line represents the bond out of the plane and towards the observer. These lines are used to represent the stereochemistry of the compound.
Answer to Problem 10.57P
The products for the given reaction including stereoisomers are shown below.
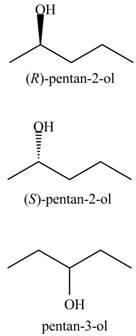
Explanation of Solution
Hydration of alkenes is one of the methods used for the formation of alcohol.
The general steps followed by hydration reaction are stated below:
• First, protonation of the alkene takes place to generate the carbocation.
• Formation of protonated alcohol.
• Deprotonation.
In this type of reaction, hydroxyl group attacks on the more substituted carbon.
The given reaction is,
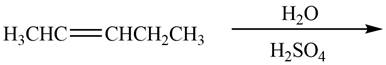
Figure 16
The products for the given reaction including stereoisomers are shown below.
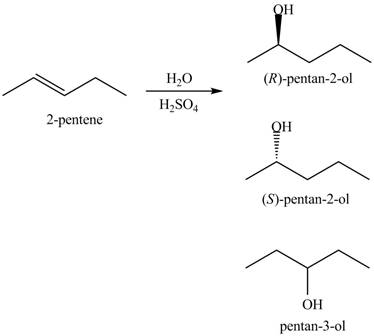
Figure 17
Stereochemistry is defined as the arrangement of molecule in three dimensional and its impact on chemical reactions. Stereochemistry for the products is shown by the dashed and wedge lines. Dashed lines represent that the bond is far away from the plane, whereas wedge line represents the bond out of the plane and towards the observer. These lines are used to represent the stereochemistry of the compound.
The products for the given reaction including stereoisomers are shown in figure 17.
Want to see more full solutions like this?
Chapter 10 Solutions
Organic Chemistry - With Access (Custom)
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





