
Concept explainers
(a)
Interpretation:
The bond strength, bond length, and bond order of
Concept introduction:
To draw the Lewis structure of the molecule there are following steps:
Step 1: Find the central atom and place the other atoms around it. The atom in a compound which has the lowest group number or lowest electronegativity considered as the central atom.
Step 2: Calculate the total number of valence electrons.
Step 3: Connect the other atoms around the central atoms to the central atom with a single bond and lower the value of valence electrons by 2 of every single bond.
Step 4: Allocate the remaining electrons in pairs so that each atom can get 8 electrons.
Step 5: Convert the lone pair into bond pair.
Bond strength is directly proportional to the bond order. While the bond length is inversely proportional to the bond order thus higher bond order means smaller bond length.
A single bond is weaker and longer than a double bond and a triple bond is most stronger and shorter.
(a)
Answer to Problem 10.61P
The bond order of the
Therefore the order of
And the order of
Explanation of Solution
Both
The total number of valence electrons of
Substitute 2 for the total number of
Lewis structure of
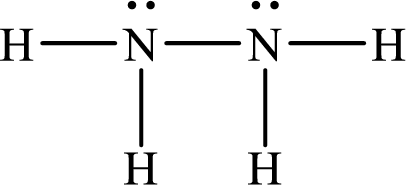
Therefore the bond order of the
The total number of valence electrons of
Substitute 2 for the total number of
Lewis structure of
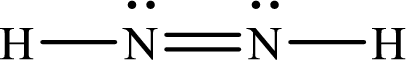
Therefore the bond order of the
The total number of valence electrons of
Substitute 2 for the total number of
Lewis structure of

Therefore the bond order of the
In Hydrazine
Thus the order of
And the order of
Bond strength is directly proportional to the bond order and bond length is inversely proportional to bond order. Hydrazine has a weakest and longest
Interpretation:
The Lewis structure for tetrazene
Concept introduction:
To draw the Lewis structure of the molecule following steps are used.
Step 1: Find the central atom and place the other atoms around it. The atom in a compound which has the lowest group number or lowest electronegativity considered as the central atom.
Step 2: Calculate the total number of valence electrons.
Step 3: Connect the other atoms around the central atoms to the central atom with a single bond and lower the value of valence electrons by 2 of every single bond.
Step 4: Allocate the remaining electrons in pairs so that each atom can get 8 electrons.
Step 5: Convert the lone pair into bond pair.
The heat of the reaction
The formula to calculate
Or,
The bond energy of reactants is positive and the bond energy of products is negative.
Answer to Problem 10.61P
The Lewis structure for Tetrazene is
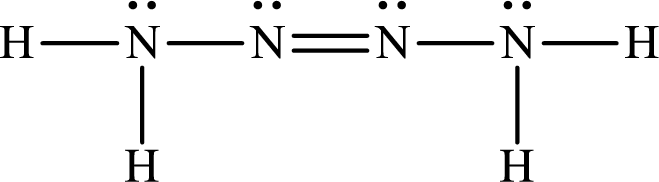
The heat of reaction
Explanation of Solution
The total number of valence electrons of
Substitute 4 for the total number of
The Lewis structure of Tetrazene is,
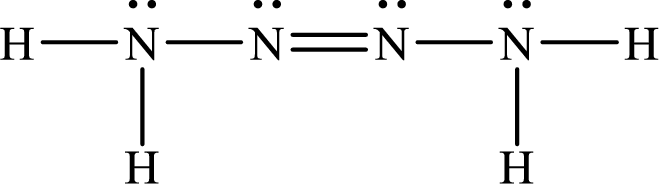
The given chemical equation for the decomposition of Tetrazene is as follows:
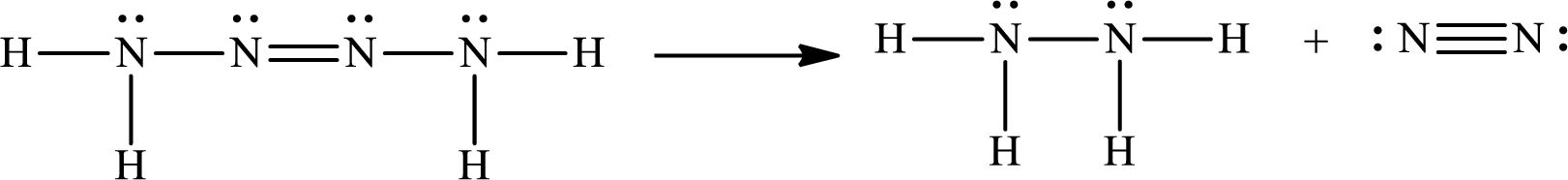
The number of broken bonds is
The number of bonds formed is
The formula to calculate the enthalpy of the given reaction is as follows:
Substitute
Only one Lewis structure is possible for tetrazene.
Want to see more full solutions like this?
Chapter 10 Solutions
Chemistry: The Molecular Nature of Matter and Change
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





