
Concept explainers
(a)
Interpretation:
Lewis structure of
Concept introduction:
The steps to draw the Lewis structure of the given molecule are as follows:
Step 1: Choose the least electronegative central metal atom and place the atoms relative to each other.
Step 2: Determine the total number of valence electron.
Step 3: Place a single electron pair between each atom and subtract 2 electrons corresponding to each of these bonds from the total number of valence electrons.
Step 4: Distribute the remaining electrons in pairs around each atom as non bonding electrons such that each atom gets a complete share of eight electrons.
(a)
Answer to Problem 10.62P
The Lewis structure of
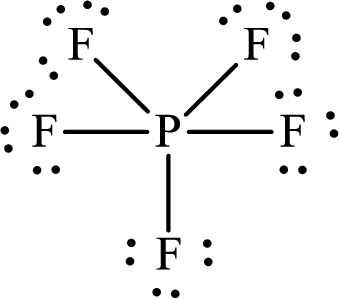
Explanation of Solution
The total number of valence electrons of
Substitute 1 for the total number of
Thus, the Lewis structure of
The molecule
(b)
Interpretation:
Lewis structure of
Concept introduction:
The steps to draw the Lewis structure of the given molecule are as follows:
Step 1: Choose the least electronegative central metal atom and place the atoms relative to each other.
Step 2: Determine the total number of valence electron.
Step 3: Place a single electron pair between each atom and subtract 2 electrons corresponding to each of these bonds from the total number of valence electrons.
Step 4: Distribute the remaining electrons in pairs around each atom as non bonding electrons such that each atom gets a complete share of eight electrons.
(b)
Answer to Problem 10.62P
The Lewis structure of
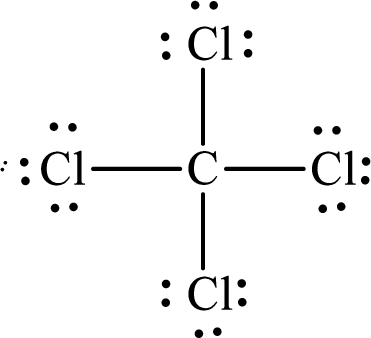
Explanation of Solution
The total number of valence electrons of
Substitute 1 for the total number of
The Lewis structure of
The molecule
(c)
Interpretation:
Lewis structure of
Concept introduction:
The steps to draw the Lewis structure of the given molecule are as follows:
Step 1: Choose the least electronegative central metal atom and place the atoms relative to each other.
Step 2: Determine the total number of valence electron.
Step 3: Place a single electron pair between each atom and subtract 2 electrons corresponding to each of these bonds from the total number of valence electrons.
Step 4: Distribute the remaining electrons in pairs around each atom as non bonding electrons such that each atom gets a complete share of eight electrons.
(c)
Answer to Problem 10.62P
The Lewis structure
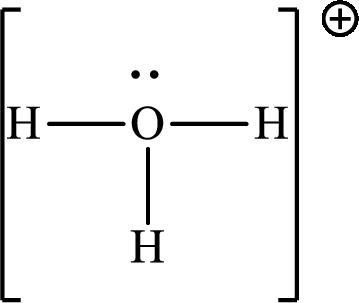
Explanation of Solution
The total number of valence electrons of
Substitute 1 for the total number of
The total number of valence electrons in
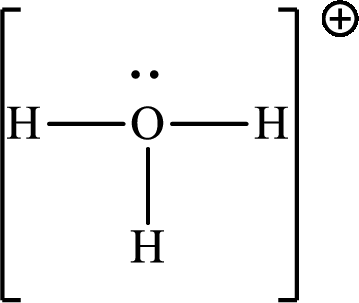
The molecular shape of
(d)
Interpretation:
Lewis structure of
Concept introduction:
The steps to draw the Lewis structure of the given molecule are as follows:
Step 1: Choose the least electronegative central metal atom and place the atoms relative to each other.
Step 2: Determine the total number of valence electron.
Step 3: Place a single electron pair between each atom and subtract 2 electrons corresponding to each of these bonds from the total number of valence electrons.
Step 4: Distribute the remaining electrons in pairs around each atom as non bonding electrons such that each atom gets a complete share of eight electrons.
(d)
Answer to Problem 10.62P
Lewis structure of
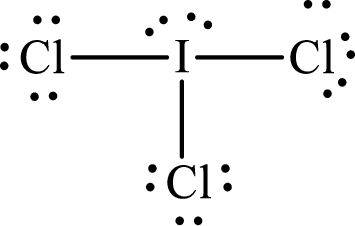
Explanation of Solution
The total number of valence electrons of
Substitute 1 for the total number of
With
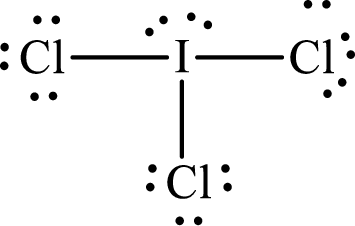
The electron group arrangement of
(e)
Interpretation:
Lewis structure of
Concept introduction:
The steps to draw the Lewis structure of the given molecule are as follows:
Step 1: Choose the least electronegative central metal atom and place the atoms relative to each other.
Step 2: Determine the total number of valence electron.
Step 3: Place a single electron pair between each atom and subtract 2 electrons corresponding to each of these bonds from the total number of valence electrons.
Step 4: Distribute the remaining electrons in pairs around each atom as non bonding electrons such that each atom gets a complete share of eight electrons.
(e)
Answer to Problem 10.62P
Lewis structure of
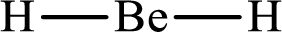
Explanation of Solution
The total number of valence electrons of
Substitute 1 for the total number of
With
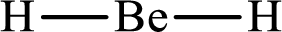
The electron group arrangement of
(f)
Interpretation:
Lewis structure of
Concept introduction:
The steps to draw the Lewis structure of the given molecule are as follows:
Step 1: Choose the least electronegative central metal atom and place the atoms relative to each other.
Step 2: Determine the total number of valence electron.
Step 3: Place a single electron pair between each atom and subtract 2 electrons corresponding to each of these bonds from the total number of valence electrons.
Step 4: Distribute the remaining electrons in pairs around each atom as non bonding electrons such that each atom gets a complete share of eight electrons.
(f)
Answer to Problem 10.62P
Lewis structure of
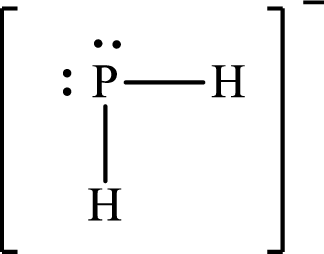
Explanation of Solution
The total number of valence electrons of
Substitute 1 for the total number of
The Lewis structure of
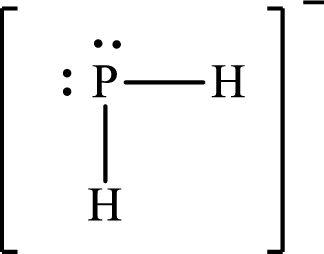
The electron group arrangement of
(g)
Interpretation:
Lewis structure of
Concept introduction:
The steps to draw the Lewis structure of the given molecule are as follows:
Step 1: Choose the least electronegative central metal atom and place the atoms relative to each other.
Step 2: Determine the total number of valence electron.
Step 3: Place a single electron pair between each atom and subtract 2 electrons corresponding to each of these bonds from the total number of valence electrons.
Step 4: Distribute the remaining electrons in pairs around each atom as non bonding electrons such that each atom gets a complete share of eight electrons.
(g)
Answer to Problem 10.62P
Lewis structure of
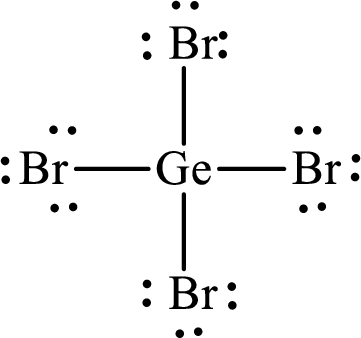
Explanation of Solution
The total number of valence electrons of
Substitute 1 for the total number of
The total number of valence electrons in
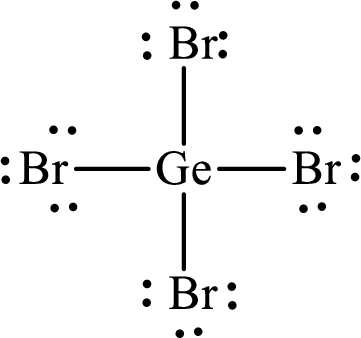
The electron group arrangement of
(h)
Interpretation:
Lewis structure of
Concept introduction:
The steps to draw the Lewis structure of the given molecule are as follows:
Step 1: Choose the least electronegative central metal atom and place the atoms relative to each other.
Step 2: Determine the total number of valence electron.
Step 3: Place a single electron pair between each atom and subtract 2 electrons corresponding to each of these bonds from the total number of valence electrons.
Step 4: Distribute the remaining electrons in pairs around each atom as non bonding electrons such that each atom gets a complete share of eight electrons.
(h)
Answer to Problem 10.62P
The Lewis structure for
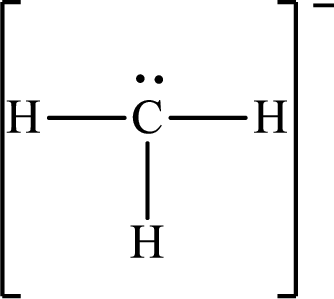
Explanation of Solution
The total number of valence electrons of
Substitute 1 for the total number of
The total number of valence electrons in
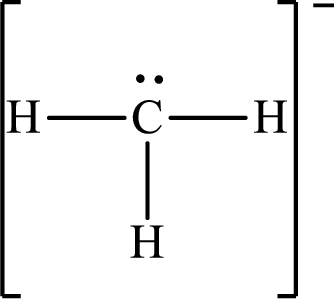
The electron group arrangement of
(i)
Interpretation:
Lewis structure of
Concept introduction:
The steps to draw the Lewis structure of the given molecule are as follows:
Step 1: Choose the least electronegative central metal atom and place the atoms relative to each other.
Step 2: Determine the total number of valence electron.
Step 3: Place a single electron pair between each atom and subtract 2 electrons corresponding to each of these bonds from the total number of valence electrons.
Step 4: Distribute the remaining electrons in pairs around each atom as non bonding electrons such that each atom gets a complete share of eight electrons.
(i)
Answer to Problem 10.62P
The Lewis structure for
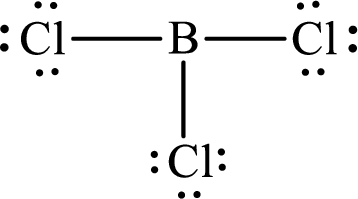
Explanation of Solution
The total number of valence electrons of
Substitute 1 for the total number of
These 24 electrons are placed such that three of these form bonding pairs and the remaining ones reside as lone pairs as shown below:
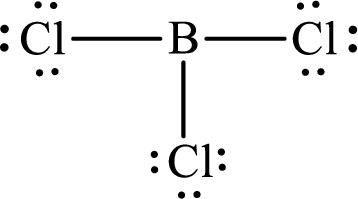
The electron group arrangement of
(j)
Interpretation:
Lewis structure of
Concept introduction:
The steps to draw the Lewis structure of the given molecule are as follows:
Step 1: Choose the least electronegative central metal atom and place the atoms relative to each other.
Step 2: Determine the total number of valence electron.
Step 3: Place a single electron pair between each atom and subtract 2 electrons corresponding to each of these bonds from the total number of valence electrons.
Step 4: Distribute the remaining electrons in pairs around each atom as non bonding electrons such that each atom gets a complete share of eight electrons.
(j)
Answer to Problem 10.62P
The Lewis structure for
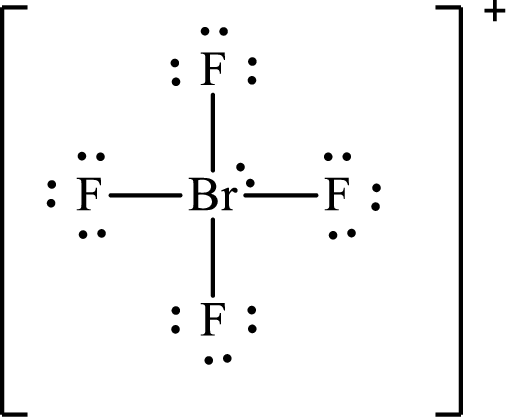
Explanation of Solution
The total number of valence electrons of
Substitute 1 for the total number of
The Lewis structure for
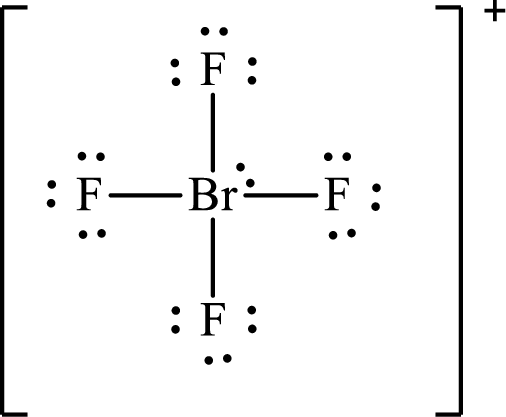
The electron group arrangement of
(k)
Interpretation:
Lewis structure of
Concept introduction:
The steps to draw the Lewis structure of the given molecule are as follows:
Step 1: Choose the least electronegative central metal atom and place the atoms relative to each other.
Step 2: Determine the total number of valence electron.
Step 3: Place a single electron pair between each atom and subtract 2 electrons corresponding to each of these bonds from the total number of valence electrons.
Step 4: Distribute the remaining electrons in pairs around each atom as non bonding electrons such that each atom gets a complete share of eight electrons.
(k)
Answer to Problem 10.62P
The Lewis structure of
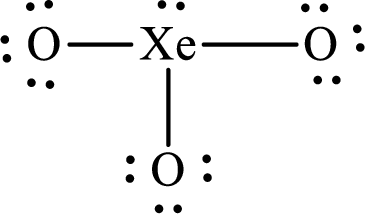
Explanation of Solution
The total number of valence electrons of
Substitute 1 for the total number of
The total number of valence electrons in
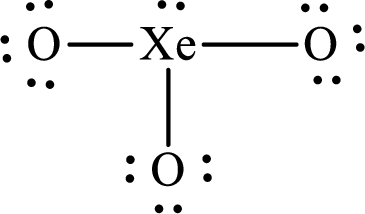
The electron group arrangement of
(l)
Interpretation:
Lewis structure of
Concept introduction:
The steps to draw the Lewis structure of the given molecule are as follows:
Step 1: Choose the least electronegative central metal atom and place the atoms relative to each other.
Step 2: Determine the total number of valence electron.
Step 3: Place a single electron pair between each atom and subtract 2 electrons corresponding to each of these bonds from the total number of valence electrons.
Step 4: Distribute the remaining electrons in pairs around each atom as non bonding electrons such that each atom gets a complete share of eight electrons.
(l)
Answer to Problem 10.62P
The Lewis structure for
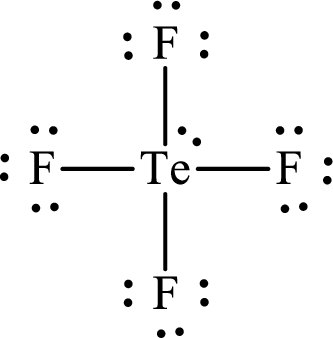
Explanation of Solution
The total number of valence electrons of
Substitute 1 for the total number of
Analogous to
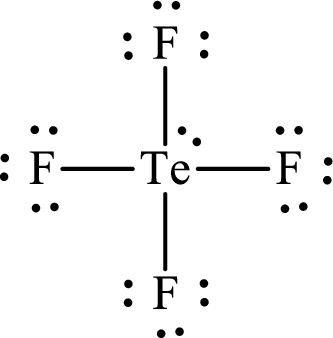
The electron group arrangement of
Want to see more full solutions like this?
Chapter 10 Solutions
CHEM 212:CHEMISTSRY V 2
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





