
Concept explainers
(a)
Interpretation:
Concept introduction:
The heat of the reaction
The formula to calculate
Or,
The bond energy of reactants is positive and the bond energy of products is negative.
(a)
Answer to Problem 10.75P
Explanation of Solution
The given chemical equation for the combustion of gaseous ethanol is as follows:
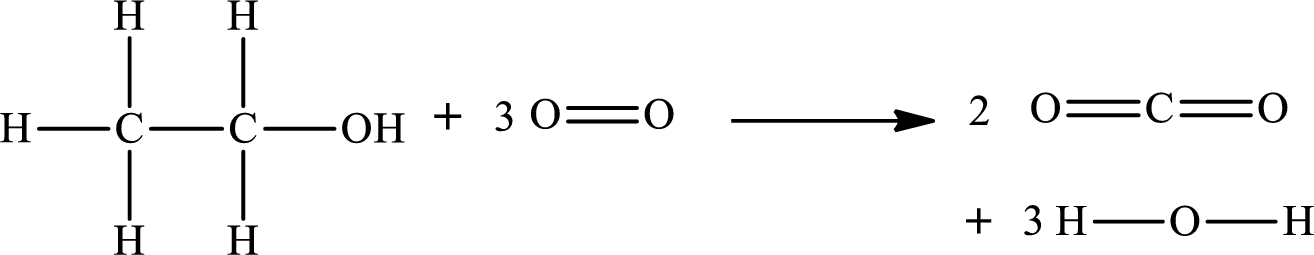
The number of broken bonds is
The number of bonds formed is
The formula to the enthalpy of the given reaction is as follows:
Substitute
The number of broken bonds is
(b)
Interpretation:
The enthalpy of reaction for the combustion of liquid ethanol is to be calculated.
Concept introduction:
The heat of the reaction
A combustion reaction is that reaction in which reactant is reacted with molecular oxygen to form the product. Heat is released and the energy is produced in the reaction. Molecular oxygen is employed as an oxidizing agent in these reactions.
(b)
Answer to Problem 10.75P
Explanation of Solution
The heat of vaporization of ethanol
Substitute
The vaporization of a liquid occurs when the intermolecular forces between the molecules break and the molecules are free to vaporize.
(c)
Interpretation:
The enthalpy of reaction for the combustion of liquid ethanol calculated from the bond energies and standard enthalpies of formation is to be compared.
Concept introduction:
The standard enthalpy of reaction is calculated by the summation of standard enthalpy of formation of the product minus the summation of standard enthalpy of formation of reactant at the standard conditions. The formula to calculate the standard enthalpy of reaction
Here, m and n are the stoichiometric coefficients of reactants and product in the balanced chemical equation.
(c)
Answer to Problem 10.75P
The enthalpy of reaction for the combustion of liquid ethanol calculated from the bond energies is close to the enthalpy of reaction for the combustion of liquid ethanol calculated from standard enthalpies of formation.
Explanation of Solution
The given chemical equation for the combustion of ethanol is as follows:
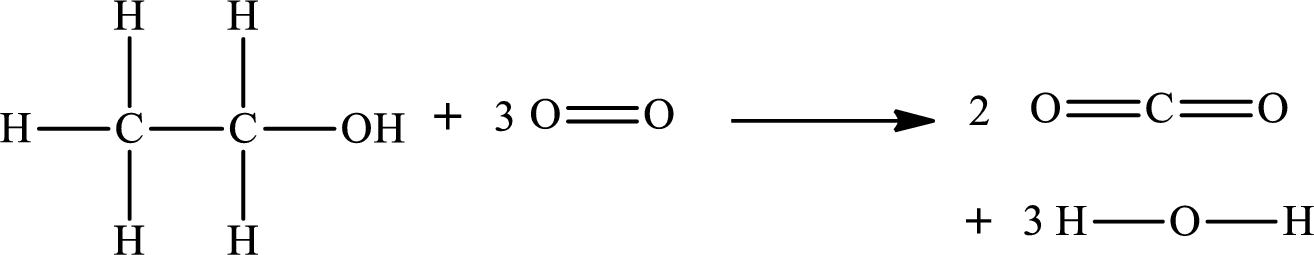
The standard state of oxygen is
The formula to calculate the standard enthalpy for the reaction
Substitute
The calculated value is close to the value calculated in part (b). Therefore the enthalpy of reaction for the combustion of liquid ethanol calculated from the bond energies and standard enthalpies of formation are in good agreement.
The enthalpy of reaction for the combustion of liquid ethanol calculated from standard enthalpies of formation is
(d)
Interpretation:
Concept introduction:
The heat of the reaction
The formula to calculate
Or,
The bond energy of reactants is positive and the bond energy of products is negative.
Lewis structure is generally considered as a simplified structure of any molecule or atom. Lewis structure for any atom or molecule depicts the valence electrons as dots around the element’s symbol present in the molecule along with the bonds that connect them. Every element tries to complete an octet except the hydrogen atom.
Every element in the Lewis structure tries to attain eight electrons in its valence shell by transfer or share of electrons. This rule is known as the octet rule.
(d)
Answer to Problem 10.75P
Explanation of Solution
The Lewis structures for the reaction of the formation of gaseous ethanol are as follows:
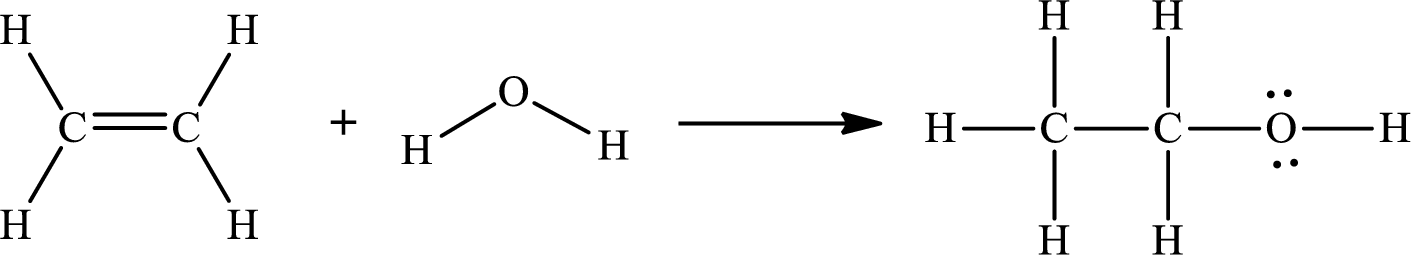
The number of broken bonds is
The number of bonds formed is
The formula to the enthalpy of the given reaction is as follows:
Substitute
The number of broken bonds is
Want to see more full solutions like this?
Chapter 10 Solutions
GEN CMB CHEM; CNCT+;ALEKS 360
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





