
Interpretation:
Titration reaction has to be written along with equivalence volume and
Concept Introduction:
Dilution formula is given as follows:
Here,
Explanation of Solution
The dilution formula is given as follows:
Substitute
Rearrange equation (2) to calculate value of
So, equivalence volume is
Equilibrium for titration reaction is given as follows:
Formula to calculate
Substitute 9.00 for
Formula to calculate
Substitute 4.00 for
Corresponding expression of
ICE table is drawn as follows:
Substitute
Simplify to obtain the value of
Until just before equivalence point there is a mixture of unionized
Substitute 4.00 for
When volume of base added is
Substitute 4.00 for
Similarly when volume of base added is
Substitute 4.00 for
Similarly when volume of base added is
Substitute 4.00 for
Similarly when volume of base added is
Substitute 4.00 for
When volume of base added is
New ICE table can be drawn as follows:
Expression of equilibrium constant for this reaction is given as follows:
Substitute
Therefore expression of equilibrium constant can be written as follows:
Simplify to obtain the value of
Substitute
When
Formula to calculate
Substitute
Formula to calculate
Substitute 3.04 for
Similarly when
Formula to calculate
Substitute
Formula to calculate
Substitute 2.04 for
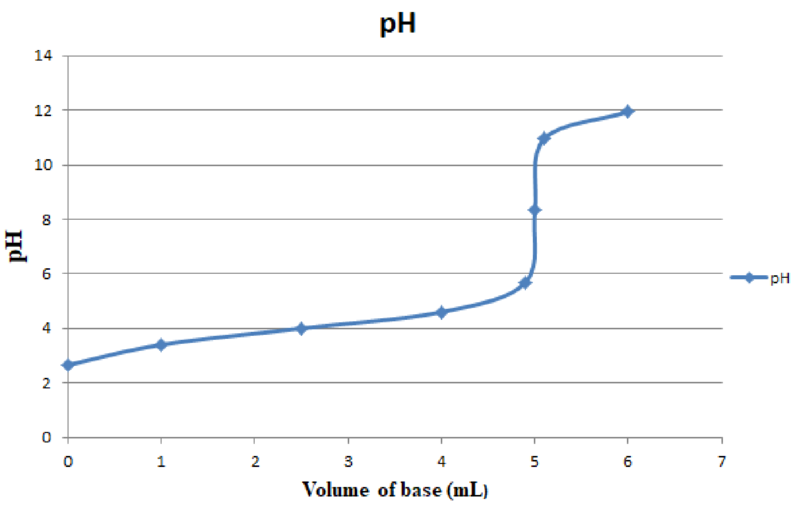
Corresponding plot of
Want to see more full solutions like this?
Chapter 10 Solutions
Exploring Chemical Analysis
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





