
Concept explainers
(a)
Interpretation:
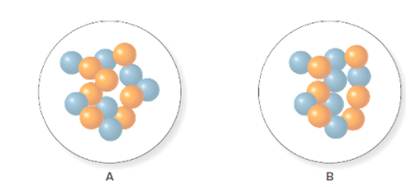
Concept Introduction:
The atomic number of an atom is equal to the number of protons in it. The atomic number is represented by Z.
Answer to Problem 20P
The atomic number for isotope of nitrogen A and B is 7.
Explanation of Solution
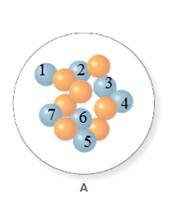
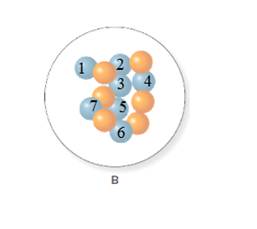
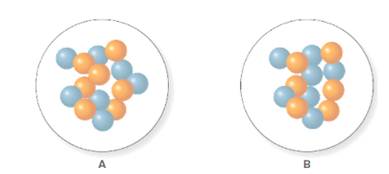
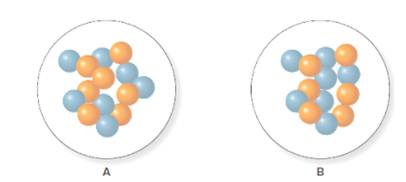
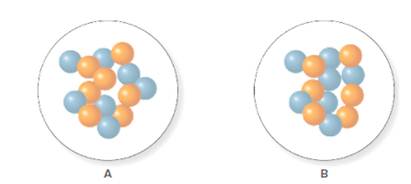
In the molecular model of fluorine, blue balls represent protons, whereas orange balls represent neutrons. Since the number of protons from given molecular model A and B is 7, the atomic number becomes 7 for A and B in both cases, as the atomic number is equal to the number of protons in that atom.
(b)
Interpretation:
The mass number for each isotope of nitrogen A and B given below should be predicted.
Concept Introduction:
The mass number is equal to the number of protons and neutrons in an atom.
Answer to Problem 20P
The mass number for isotope of nitrogen A and B is 14 and 13 respectively.
Explanation of Solution
From the molecular model of an isotope of nitrogen, the number of neutrons can be counted. Thus, the number of neutrons in A and B is 7 and 6 respectively.
As
For A and B, the mass number will be calculated as follows:
(c)
Interpretation:
The number of protons for each isotope of nitrogen A and B given below should be predicted.
Concept Introduction:
The number of protons is equal to the atomic number of that atom.
Answer to Problem 20P
The number of protons in isotope of nitrogen A and B is 7.
Explanation of Solution
As the number of protons is equal to the atomic number in an atom. The atomic number of an isotope of nitrogen of A and B is 7. Thus, the number of protons in A and B will be 7.
(d)
Interpretation:
The number of neutrons for each isotope of nitrogen A and B given below should be predicted.
Concept Introduction:
The mass number is the sum of all the protons and neutrons present in an atom. Thus, the number of neutrons can be calculated simply by subtracting the number of protons from the mass number of that atom.
Answer to Problem 20P
The number of neutrons in A and B is 7 and 6 respectively.
Explanation of Solution
For A the mass number and number of protons are 14 and 7 respectively. Thus, the number of neutrons in A will be calculated as follows:
For B the mass number and number of protons are 13 and 7 respectively. Thus, the number of neutrons in B will be calculated as follows:
(e)
Interpretation:
The isotope symbol for each isotope of nitrogen A and B given below should be predicted.
Concept Introduction:
Isotopes are the compounds having the same atomic number but different
To write an isotope symbol atomic number (Z) is written on the lower left side and atomic mass(A) is written on the upper left side of an element.
Answer to Problem 20P
The isotope symbol for A and B is
Explanation of Solution
For A, the mass number and atomic number are 14 and 7 respectively. Thus, the isotope symbol for A will be represented as follows:
For B, the mass number and atomic number are 13 and 7 respectively. Thus, the isotope symbol for B will be represented as follows:
Want to see more full solutions like this?
Chapter 10 Solutions
GENERAL ORGANIC & BIOCHEMISTRY >ACCESS<
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning





