
Concept explainers
State the number of valence electrons, bonding pairs, and lone pairs in each of the following Lewis structures: (10.1) 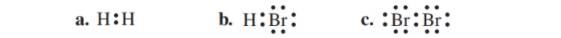
(a)
Interpretation:
Interpret number of valence electron, bond pair and lone pair in the given Lewis structure.
Concept Introduction:
Valence electrons are the electrons present in the outermost orbital or shell of an atom which participates in the formation of bond with another atom.
The bond formed between two atoms by sharing of electrons is known as covalent bond. A single chemical bond is formed by sharing of 2 electrons while double bond is formed by sharing of 4 and triple bond is formed by sharing of 6 electrons.
Total number of valence electron can be determined by adding all the electrons present in the outermost shell of each atom present in a molecule.
For example, inHxOy
Total number of valence electron = number of H (valence electron of H) + number of O (valence electron of O)
Answer to Problem 59UTC
Total number of valence electron = 2
Bond pair = 1
Lone pair = 0
Explanation of Solution
The given Lewis structure is as follows:
Total number of valence electron = number of H (valence electron of H)
Total number of valence electron = 2
Bond pair = 1
Lone pair = 0
(b)
Interpretation:
Interpret number of valence electron, bond pair and lone pair in given Lewis structure.
Concept Introduction:
Valence electrons are the electrons present in the outermost orbital or shell of an atom which participates in the formation of bond with another atom.
The bond formed between two atoms by sharing of electrons is known as covalent bond. A single chemical bond is formed by sharing of 2 electrons while double bond is formed by sharing of 4 and triple bond is formed by sharing of 6 electrons.
Total number of valence electron can be determined by adding all the electrons present in the outermost shell of each atom present in a molecule.
For example, inHxOy
Total number of valence electron = number of H (valence electron of H) + number of O (valence electron of O)
Answer to Problem 59UTC
Total number of valence electron = 8
Bond pair = 1
Lone pair = 3
Explanation of Solution
The given Lewis structure is of HBr.
Total number of valence electron = number of H (valence electron of H) + number of Br (valence electron of Br)
Total number of valence electron = 1 (1) + 1 (7) = 8
Bond pair = 1
Lone pair = 3
(c)
Interpretation:
Interpret number of valence electron, bond pair and lone pair in Br-Br
Concept Introduction:
Valence electrons are the electrons present in the outermost orbital or shell of an atom which participates in the formation of bond with another atom.
The bond formed between two atoms by sharing of electrons is known as covalent bond. A single chemical bond is formed by sharing of 2 electrons while double bond is formed by sharing of 4 and triple bond is formed by sharing of 6 electrons.
Total number of valence electron can be determined by adding all the electrons present in the outermost shell of each atom present in a molecule.
For example, inHxOy
Total number of valence electron = number of H (valence electron of H) + number of O (valence electron of O)
Answer to Problem 59UTC
Total number of valence electron = 2 (7) = 14
Bond pair = 1
Lone pair = 6
Explanation of Solution
The given Lewis structure is of Br2.
Total number of valence electron = number of Br (valence electron of Br)
Total number of valence electron = 2 (7) = 14
Bond pair = 1
Lone pair = 6
Want to see more full solutions like this?
Chapter 10 Solutions
Basic Chemistry Plus Mastering Chemistry With Pearson Etext -- Access Card Package (6th Edition)
- A 27 g aluminum foil pan is used to roast vegetables. The pan is put into a cold oven at 22 oC . How much energy in cal is absorbed by the pan after cooking at 232 oC (450. oF) for 25 minutes?arrow_forwardCalculate the value of ∆ H° for the following equation using the ∆ Hf° values CCl4(l) = -135.4 kJ/mol and CCl4(g) = -95.98 kJ/mol CCl4(l) → CCl4(g)arrow_forwardAnswer the question attached with correct sig digs: Calculate ?rH for the reaction 2 NOCI(g)---> N2(g) + O2(g) + Cl2(g) using the following reactions: ½N2(g) + O2(g) →→→ NO(g) ?rH= 90.3 kJ/mol NO(g) + Cl2(g) →→→ NOCI(g) ?rH=-38.6 kJ/molarrow_forward
- The plastic known as PETE (polyethyleneterephthalate) is used to make plastic soft drink bottles and containers for salad dressing, shampoos, and dishwashing liquids. PETE is a polymer of terephthalic acid and ethylene glycol. Today, PETE is the most widely recycled of all the plastics. After it is separated from other plastics, PETE can be used in polyester fabric, door mats, and tennis ball containers. In 2015, 1.84×109 lb1.84×109 lb of PETE bottles were recycled in the U.S. The density of PETE is 1.38 g/mLg/mL. What volume, in liters, of PETE bottles were recycled in 2015 in the U.S.? Express your answer to three significant figures.arrow_forwardThe plastic known as PETE (polyethyleneterephthalate) is used to make plastic soft drink bottles and containers for salad dressing, shampoos, and dishwashing liquids. PETE is a polymer of terephthalic acid and ethylene glycol. Today, PETE is the most widely recycled of all the plastics. After it is separated from other plastics, PETE can be used in polyester fabric, door mats, and tennis ball containers. In 2015, 1.84×109 lb1.84×109 lb of PETE bottles were recycled in the U.S. The density of PETE is 1.38 g/mLg/mL. Suppose a landfill holds 2.6×107 L2.6×107 L of recycled PETE. If all the PETE bottles recycled in 2015 in the U.S. were placed instead in landfills, how many landfills would be needed? Express your answer as an integer.arrow_forwardThe plastic known as PETE (polyethyleneterephthalate) is used to make plastic soft drink bottles and containers for salad dressing, shampoos, and dishwashing liquids. PETE is a polymer of terephthalic acid and ethylene glycol. Today, PETE is the most widely recycled of all the plastics. After it is separated from other plastics, PETE can be used in polyester fabric, door mats, and tennis ball containers. In 2015, 1.84×109 lb1.84×109 lb of PETE bottles were recycled in the U.S. The density of PETE is 1.38 g/mLg/mL. How many kilograms of PETE bottles were recycled in 2015 in the U.S.? Express your answer to three significant figures.arrow_forward
- The plastic known as PETE (polyethyleneterephthalate) is used to make plastic soft drink bottles and containers for salad dressing, shampoos, and dishwashing liquids. PETE is a polymer of terephthalic acid and ethylene glycol. Today, PETE is the most widely recycled of all the plastics. After it is separated from other plastics, PETE can be used in polyester fabric, door mats, and tennis ball containers. In 2015, 1.84×109 lb1.84×109 lb of PETE bottles were recycled in the U.S. The density of PETE is 1.38 g/mLg/mL. If 2.74×109 kg2.74×109 kg of PETE bottles were sold in 2015, what percentage of those bottles were recycled? Express your answer to three significant figures.arrow_forward3. A. Draw and label an energy diagram for a slow endothermic reaction B. A weather balloon contains 222 L of helium gas at 20.8 °C and 754 mm Hg. What is the volume in liters of the balloon when it rises to an altitude where the pressure is 552 mm Hg and the temperature is -40.0 °C C. Draw two different Lewis structures for C2H4O2, connecting the atoms differently in your two structures. Label the shape of each carbon atom in both structures.arrow_forwardTABLE A-V (H2) Gf= 0 kJ/mol (CO) Gf= -137.15 kJ/mol (HCHO) Gf= -113 kJ/molarrow_forward
- Calculate ΔHorxn for SiO2(s) + 4 HF(g) --> SiF4(g) + 2 H2O(l) ΔHof [SiO2 (s)] = = −910.9 kJ/mol ΔHof [HF (g)] = −273 kJ/mol ΔHof [SiF4 (g)] = −1,614.9 kJ/mol ΔHof [H2O (l)] = −285.840 kJ/molarrow_forwardLiquid menthonal (CH4O) which is used as a cooking fuel, burns with oxygen gas to produce the gases carbon dioxide and water. The reaction produces 363 kJ of heat per mole of methonol. a) write a balanced equation for the reaction includig the heat of the reaction b) is the reaction endothermic or exothermic? c) How many moles of O2 must react with 0.450 mole of CH4O? d) How many grams of CO2 are produced when 78.0 g of Ch4O reacts?arrow_forwardThe heat of neutralization, Hneut, can be defined as the amount of heat released (or absorbed), q, per mole of acid (or base) neutralized. Hneut for nitric acid is -52 kJ/mol HNO3. At 27.3C, 50.00 mL of 0.743M HNO3 is neutralized by 1.00 M Sr(OH)2 in a coffee-cup calorimeter. (a) How many mL of Sr(OH)2 were used in the neutralization? (b) What is the final temperature of the resulting solution? (Use the assumptions in Question 11.)arrow_forward
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning


