
Interpretation:
The major product that is expected has to be drawn when the given compound is treated with ethoxide to give an E2 reaction.
Concept Introduction:
Elimination reactions are the one in which the groups are lost and the saturated bonds are converted to unsaturated bonds. Usually the substitution reaction compete with elimination reaction.
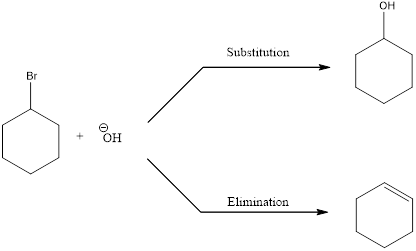
In elimination reaction, the beta proton is removed together with the leaving group to form a double bond.
E2 reaction proceeds through a single step without any formation of intermediates. The base abstracts a proton form the substrate and the loss of leaving group also happens resulting in the formation of double bond. E2 stands for bimolecular elimination.
The proton can be abstracted by the base in two different ways leading to a regiochemical outcome. In the given reaction below, two
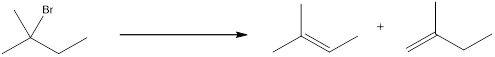
The two alkenes that are formed can be given different names. If the alkene is more substituted means it is known as Zaitsev product, and if the alkene formed is less substituted means it is known as Hofmann product. Generally more substituted product is the major product (Zaitsev product).
If the reaction is performed with a sterically hindered base, the major product will be less substituted alkene (Hofmann product). The regiochemical outcome can be decided by choosing the base. Some of the sterically hindered bases are,

Want to see the full answer?
Check out a sample textbook solution
Chapter 10 Solutions
Organic Chemistry As a Second Language: First Semester Topics
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





