
(a)
Interpretation:
The stereo isomeric product should be given when the
Concept introduction:
An alkene undergoes an oxidation reaction with Osmium tetra oxide and followed by hydrolysis to give a cis 1, 2
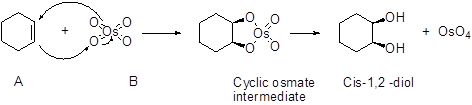
(b)
Interpretation:
The stereo isomeric product should be given when the alkenes reaction with osmium tetroxide followed by hydrogen peroxide hydrolysis.
Concept introduction:
An alkene undergoes an oxidation reaction with Osmium tetra oxide and followed by hydrolysis to give a cis 1, 2 diol for example,
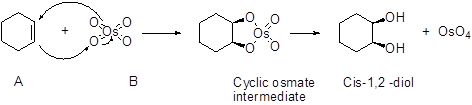
(c)
Interpretation:
The stereo isomeric product should be given when the alkenes reaction with osmium tetroxide followed by hydrogen peroxide hydrolysis.
Concept introduction:
An alkene undergoes an oxidation reaction with Osmium tetra oxide and followed by hydrolysis to give a cis 1, 2 diol for example,
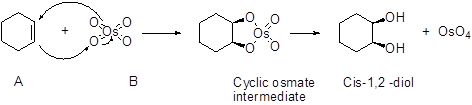
(d)
Interpretation:
The stereo isomeric product should be given when the alkenes reaction with osmium tetroxide followed by hydrogen peroxide hydrolysis.
Concept introduction:
An alkene undergoes an oxidation reaction with Osmium tetra oxide and followed by hydrolysis to give a cis 1, 2 diol for example,
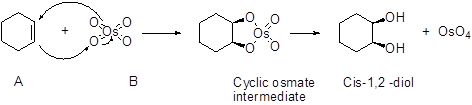
Want to see the full answer?
Check out a sample textbook solution
Chapter 10 Solutions
Organic Chemistry (8th Edition)
- A haloalkane reacts with a strong base to yield only one alkene product. Select the possible structures that can give this result from Figure 9. * A B C Darrow_forwardDraw the major products obtained from the reaction of one equivalent of HCl with the following compounds. For each reaction, indicate the kinetic andthermodynamic products. a. 2,3-dimethyl-1,3-pentadiene b. 2,4-dimethyl-1,3-pentadienearrow_forwardWhat reagents would you use to prepare the next alkenearrow_forward
- Which reaction intermediate is formed when 4-methylcyclohexene reacts with Br2 dissolved in CCl4arrow_forwardPick the reactant or solvent in each part that gives the faster elimination reaction.a. reaction of -OH with 1-chloro-1-methylcyclohexane or 1-chloro-3-methylcyclohexaneb. reaction of H2O with CH3CH(Cl)CH2CH3 or (CH3)2C(Cl)CH2CH3c. reaction of (CH3)3CCl with -OH in H2O or DMSOarrow_forwardWhat is the reaction when trans-2-butene reacts with 1.KMnO4/-OH in cold 2. hotKMnO4/-OH 3.HBr in the presence of peroxidearrow_forward
- 1. Which reaction conditions would be best for turning ethene into ethanol? a.H2O, HBr b. CH3OH, H2SO4 c.CH3OH, HBR d. H2O, H2SO4 2. Which alkene would be non-regioselective in a reaction with HBr? a. 2-ehyl-2-methyl-2-butene b.2-ethyl-3-methyl-2-butene c. 2,3-dimethyl-2-butene d.1,2-dimethyl-2-butenearrow_forwardConsider a reaction where cis-but-2-ene is treated with a peroxy acid followed by OH- /H20. Draw the structure of one product that is formed in the reaction, including correct stereochemistry.arrow_forward1. Using Br2 in C2H4Br2 will result in HBr and ______. a. C2H3Cl3 b. C2H4Cl3 c. C2H2Cl3 d. none of the above 2. How many halogenation are posible in propane? a. 3 b. 8 c. 6 d. 10 3.Sulfonation of pentane will result in ________ and water. a. C5H11SO3H b. C5H12SO3H c. C5H14SO3H d. none of the above 4.Nitration of hexane will result in ________ and water. a. C6H13SO3H b. C6H15NO2 c. C6H13NO2 d. C6H14NO2 5.How many moles of O2 in heating a C12H26 (dodecane) a. 27 b. 37 c. 24 d. none of the abovearrow_forward
- During the further development of other prostaglandin-like molecules, it is necessary to replace the cyclopentane ring with the cyclohexene as shown in the reaction scheme below. (Deuterium (D) is a heavier isotope of hydrogen) a) Draw the most stable conformation of the starting material 8 and explain why this is the most stable conformation. b) Draw the reaction mechanism for how 9 is formed from 8 and explain why 10 and 11 are not formed if one assumes the E2 mechanism.arrow_forward1.Label kinetic product 2.Label the thermodynamic product 3.Predict the four products from reaction of alkene with one equivalent HBrarrow_forwardWhat is the major product obtained from hydroboration–oxidation of the following alkenes? a. 2-methyl-2-butene b. 1-methylcyclohexenearrow_forward

