
(a)
Interpretation:
The reagent that is needed to convert to ![]() each of the compound needs to be identified.
each of the compound needs to be identified.
Concept introduction:
Addition of H2O to an
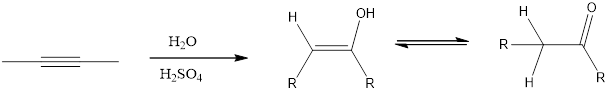
(b)
Interpretation:
The reagent that is needed to ![]() convert to each of the compound needs to be identified.
convert to each of the compound needs to be identified.
Concept introduction:
Addition of 1. R2BHand 2. H2O2, OH- reagent to an alkyne is a hydroboration-oxidation reaction to form the keto product as shown below in the fig. Addition of borane forms an organoborane. Oxidation with basic H20 2 forms an enol. Tautomerization of the enol forms a carbonyl compound. The overall result is addition of H2O to a triple bond.
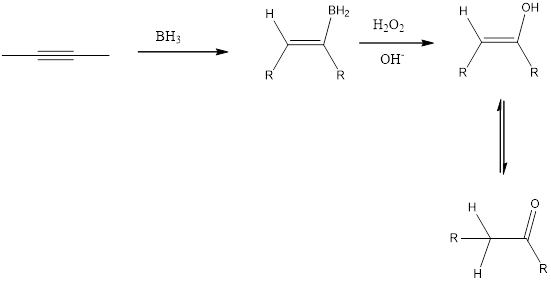
(c)
Interpretation:
The reagent that is needed to convert to ![]() each of the compound needs to be identified.
each of the compound needs to be identified.
Concept introduction:
Reaction between the 3-hexyne and HBr reagent is an example of electrophilic addition. The alkynes undergoes addition reaction because of pi bond in them. The electrophilic end of the reagent is attracted to the electron rich triple bond. It is a sequential reaction in which the addition of the reagent to alkyne to alkene which then addition of one more equivalent reagent to form the four new bond as
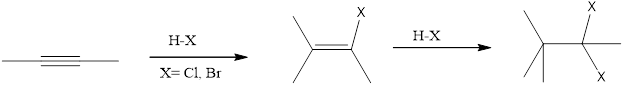
(d)
Interpretation:
The reagent that is needed to ![]() convert to each of the compound needs to be identified.
convert to each of the compound needs to be identified.
Concept introduction:
Terminal alkynes are readily converted into acetylide anions using the strong bases such as NaNH2 and NaH. These anions are strong nucleophiles, capable of reacting with electrophiles such as
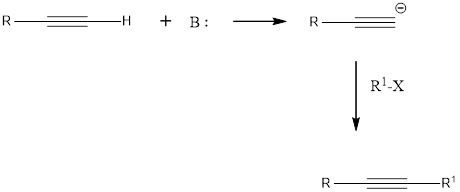
Want to see the full answer?
Check out a sample textbook solution
Chapter 11 Solutions
Connect Access Card For Organic Chemistry
- Draw the products formed when CH3CH2C=C Na+ reacts with each compound. a. CH3CH2CH2Br b.(CH3)2CHCH2CH2Cl c.(CH3CH2)3CCl d.BrCH2CH2CH2CH2OH e. ethylene oxide followed by H2O f.propene oxide followed by H2Oarrow_forwardDraw the products formed when A or B is treated with each reagent. In some cases, no reaction occurs.a. NaBH4, CH3OHb. [1] LiAlH4; [2] H2Oc. [1] CH3MgBr (excess); [2] H2Od. [1] C6H5Li (excess); [2] H2Oe. Na2Cr2O7, H2SO4, H2Oarrow_forwardBicyclo[2.2.1]heptan-7-one + PCC (in CH₂Cl₂) => A.) Bicyclo[2.2.1]heptan-7-ol B.) Bicyclo[2.2.1]heptanoic acid C.) Cyclohexanecarbaldehyde D.) All the given choices are possible products E.) No reactionarrow_forward
- (a) Give an acceptable name for each compound, (b) Draw the organic products formed when A or B is treated with each reagent: [1] H3O+; [2] −OH, H2O; [3] CH3CH2CH2MgBr (excess), then H2O; [4] LiAlH4, then H2O.arrow_forwardWhat product is formed when acetic acid is treated with each reagent: (a) CH3NH2; (b) CH3NH2, then heat; (c) CH3NH2 + DCC?arrow_forward(a) Give an acceptable name for compound A. (b) Draw the organic products formed when A is treated with each reagent: [1] H3O+; [2] −OH, H2O; [3] CH3CH2CH2MgBr (excess), then H2O; [4] LiAlH4, then H2O.arrow_forward
- Identify the missing reagents a-f in the following scheme:arrow_forwardShow how you might use the Williamson ether synthesis to prepare each ether. (a) (b)arrow_forwardDevise a synthesis of each substituted cyclopropane. Use acetylene (HC=CH) as a starting material in part (a) and cyclohexanone as a starting material in part (b). You may use any other organic compounds and any needed reagents.arrow_forward
- Match the ff reagents: a. Br₂ in CH₂Cl₂ b.CrO₃ , H₂SO₄, acetone c. concentrated HCl with ZnCl₂ d. aqueous FeCl₃ e. aqueous NaHCO₃ f. ammoniacal AgNO₃ 1. 4-bromophenol from benzene 2. hexane from 1-hexene 3. 1-hexanol from hexanal 4. hexane from hexanoic acid 5. 2-hexanol from 2-methyl-2-hexanol 6. 1-hexene from 1-hexynearrow_forwardFor the following reaction scheme, match the correct reagent to each reaction (A, B, C, D and E).arrow_forwardAnswer each question using the ball-and-stick model of compound A. Draw a constitutional isomer that contains an ether and give its IUPACname. Draw the products formed (including stereochemistry) when A istreated with each reagent: [1] NaH; [2] H2SO4; [3] POCl3, pyridine; [4]HCl; [5] SOCl2, pyridine; [6] TsCl, pyridinearrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

