
Concept explainers
Answer the following questions about erlotinib and phomallenic acid C. Erlotinib, sold under the trade name Tarceva, was introduced in 2004 for the treatment of lung cancer. Phomallenic acid C is an inhibitor of bacterial fatty acid synthesis.
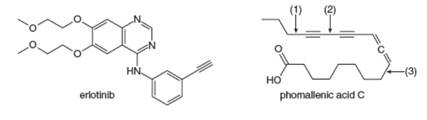
a. Which
b. What orbitals are used to form the shortest
c. Which H atom in phomallenic acid C is most acidic.
d. How many
e. Rank the labeled bonds in phomallenic acid C in order of increasing bond strength.
Want to see the full answer?
Check out a sample textbook solution
Chapter 11 Solutions
Connect Access Card For Organic Chemistry
Additional Science Textbook Solutions
General, Organic, and Biological Chemistry: Structures of Life (5th Edition)
General, Organic, & Biological Chemistry
Chemistry
Chemistry In Context
Elementary Principles of Chemical Processes, Binder Ready Version
Fundamentals of Heat and Mass Transfer
- Is p-methylphenol more acidic than phenol? Why or why not?arrow_forwardAmino acids such as glycine are the building blocks of large molecules called proteins that give structure to muscle, tendon, hair, and nails. a. Explain why glycine does not actually exist in the form with all atoms uncharged, but actually exists as a salt called a zwitterion. b. What product is formed when glycine is treated with concentrated HCl? c. What product is formed when glycine is treated with NaOH?arrow_forwardWhat is the conjugate acid of H2PO4-1? Select one: a. H3PO4 b. HPO4-2 c. PO4-3 d. H4PO4+1arrow_forward
- 16-26 The p/fb of amphetamine is approximately 3.2 Amphetamine (a) Which form of amphetamine (the free base or its conjugate acid) would you expect to be present at pH 1.0, the pH of stomach acid? (b ) Which form of amphetamine would you expect to be present at pH 7.40, the pH of blood plasma?arrow_forwardThioglycolic acid, HSCH2CO2H, a substance used in depilatory agents (hair removers) has pKa = 3.42. What is the percent dissociation of thioglycolic acid in a buffer solution at pH = 3.0?arrow_forwardThe following pKa values have been measured. Explain why a hydroxyl group in the para position decreases the acidity while a hydroxyl group in the meta position increases the acidity.arrow_forward
- Amino acids such as glycine are the building blocks of large molecule.called proteins that give structure to muscle, tendon, hair, and nails.a.Explain why glycine does not actually exist in the form with all atoms uncharged, but actually exists as a salt called a zwitterion. b.What product is formed when glycine is treated with concentrated HCl? c. What product is formed when glycine is treated with NaOH?arrow_forwardHCN HPO4 2- CN-1 + HPO4-1 Which specie is bronsted base?arrow_forward1. what priority functional group of ff organic compound? a. carboxyl b. hydroxide c. hydroxyl d. carbonyl 2. what group does the ff organic compound belong? a. azo b. diazo c. aromatic d. organosulfur 3. what group does the ff organic compound belong? a. azo b. amines c. diazo d. nitrilesarrow_forward
- Rank the following compounds in their correct order of acidity. 1=Most acidic and 4=least acidic.arrow_forwardIn each pair of compounds, select the stronger base, and explain your choice.(a) HOCH2CH2NH2 or CH3CH2NH2 (b) PhNH2 or PhCH2NH2arrow_forwardName the following molecules, then rank their basicities in decreasing order (1 = most basic, 3 = least basic).arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning



