
(a)
Interpretation:
The balanced equation to represent the complete combustion of the given compound is to be stated.
Concept introduction:
Alkane is made of only carbon and hydrogen atoms. These atoms form a bond with each other and with other atoms through the sharing of electrons. They do not completely donate their electrons to other atoms while forming a bond. A general complete combustion reaction of
Answer to Problem 11.61E
The balanced equation to represent the complete combustion of the given compound is shown below as,
Explanation of Solution
The given molecule is propane. The chemical formula of butane is
A general complete combustion reaction of alkanes is shown below as,
Where,
•
The number of carbon atoms in propane is three.
Therefore, the balanced equation to represent the complete combustion of the given compound is shown below as,
The balanced equation to represent the complete combustion of the given compound is shown below as,
(b)
Interpretation:
The balanced equation to represent the complete combustion of the given compound is to be stated.
Concept introduction:
Alkane is made of only carbon and hydrogen atoms. These atoms form a bond with each other and with other atoms through the sharing of electrons. They do not completely donate their electrons to other atoms while forming a bond. A general complete combustion reaction of alkanes is shown below as,
Answer to Problem 11.61E
The balanced equation to represent the complete combustion of the given compound is shown below as,
Explanation of Solution
The given molecule is shown below as,
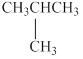
Figure 1
The chemical formula of the given compound is
A general complete combustion reaction of alkanes is shown below as,
Where,
•
The number of carbon atoms in the given compound is four.
Therefore, the balanced equation to represent the complete combustion of the given compound is shown below as,
The reaction is further simplified as,
The balanced equation to represent the complete combustion of the given compound is shown below as,
(c)
Interpretation:
The balanced equation to represent the complete combustion of the given compound is to be stated.
Concept introduction:
Alkane is made of only carbon and hydrogen atoms. These atoms form a bond with each other and with other atoms through the sharing of electrons. They do not completely donate their electrons to other atoms while forming a bond. A general complete combustion reaction of cycloalkanes is shown below as,
Answer to Problem 11.61E
The balanced equation to represent the complete combustion of the given compound is shown below as,
Explanation of Solution
The given molecule is shown below as,
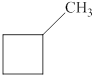
Figure 2
The chemical formula of the given compound is
A general complete combustion reaction of cycloalkanes is shown below as,
Where,
•
The number of carbon atoms in the given compound is five.
Therefore, the balanced equation to represent the complete combustion of the given compound is shown below as,
The above reaction is further simplified as,
The balanced equation to represent the complete combustion of the given compound is shown below as,
Want to see more full solutions like this?
Chapter 11 Solutions
Chemistry for Today: General, Organic, and Biochemistry
- Organic Compound: Isooctane A. Give the use of the organic compound in everyday life B. Effects to humans and other living things C. Precautions in using the compoundarrow_forwardGive the IUPAC names of the ff. organic compoundsarrow_forwardDraw the structural formulas corresponding to each of the following IUPAC names: a.ethylcyclobutane b.1, 1, 2, 5-tetramethylcyclohexane c.1-butyl-3-isopropylcyclopentanearrow_forward
- Hydrocarbons are generally polar. They are generally soluble in organic solvents. a. if the first statement is true and the second statement is false. b. If the first statement is false and the second statement is true. c. If both statements are false. d. If both statements are true.arrow_forwardWhat reagents are used in the esterification of Alcohols and Phenols? a.Write the reaction involved in Primary Alcohol (Ethanol) and Acetyl Chloride b. Write the reaction involved in Phenol and Acetyl Chloridearrow_forwardDraw the structure of cyclohexane.arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning





