
Suppliers of radioisotopically labeled compounds usually provide each product as a mixture Of labeled and unlabeled material. Unlabeled material is added deliberately as a carrier, partly because the specific activity of the carrier-free product is too high to be useful and partly because the product is more stable at lower specific activities. Using the radioactive decay law, calculate the
following.
a. The specific activity Of carrier-free [22P]-orthophosphate, in mCi/mmol.
b. The fraction Of H atoms that are radioactive in a preparation Of uniform-label [3H]-leucine, provided at 10 mCi/mmol.
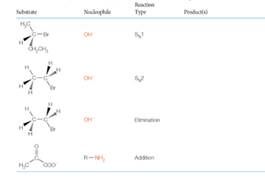
Predict the product(s) of the following reactions:
Learn your wayIncludes step-by-step video

Chapter 11 Solutions
Biochemistry: Concepts and Connections (2nd Edition)
Additional Science Textbook Solutions
Fundamentals of General, Organic, and Biological Chemistry (8th Edition)
Biology: Life on Earth
Basic Chemistry (5th Edition)
Concepts of Genetics (11th Edition)
Biological Science (6th Edition)
- If the phosphorus atom in 3-phosphoglycerate is radioactively labeled, where will the label be when the reaction that forms 2-phosphoglycerate is over?arrow_forwardAnswer the following questions yes or no (Y/N): Is the michaelis-menten equation valid under experimental conditions? Is the michaelis-menten equation only valid for reactions that are at equilibrium? Is extrapolation to Vm inaccurate, but Km is accurately determined in the michaelis-menten equation? Is the michaelis-menten equation nonlinear? Is extrapolation to Vm inaccurate, and therefore Km cannot be accurately determined in the michaelis-menten equation?arrow_forwardProvide a brief definition and a description of the significance of the following terms in NMR spectroscopy (1) CW-NMR spectroscopy (2) the nuclear Overhauser effect (3) the free induction decay(4) a 90o pulse(5) J coupling(6) distance geometry(7) intermediate exchange(8) SAR by NMR(9) the Larmour frequency(10) residual dipolar couplingsarrow_forward
- The Ksp values of silver chromate Ag2CrO4 and silver iodate Ag(IO3) are given below. Ag2CrO4 Ag(IO3) Ksp 1.12 x 10-12 3.17 x 10-8 Based on these Ksp values, which of the following is true? Choose one option only. Options: a. In the solution consisting of 1.00 x10-4 M Ag+ and 5.00 x10-5 M CrO42-, Ag2CrO4 precipitate will form. b. In the solution consisting of 1.0 x10-4 M Ag+ and 1.0 x10-4 M IO3-, Ag(IO3) precipitate will form. c. In pure water, the solubility of Ag2CrO4 is lower than the solubility of Ag(IO3). d. In the solution consisting of 0.200 M CrO42- and 0.200 M IO3-, Ag2(CrO4) will precipitate first if we add Ag+ ions gradually into the above mixture.arrow_forwardThe formation constants at 25°C for Fe(CN)4-6 and Fe(EDTA)2– are 1.00 x 1037 and 2.10 x 1014, respectively. Answer the questions below. 1) Calculate K under standard conditions for the reaction Fe(EDTA)2−(aq) + 6CN−(aq) ----> Fe(CN)4−6(aq) + EDTA4−(aq) 2) Calculate ΔG° for the reaction. (kJ/mol)arrow_forwardAn enzyme (molecular weight= 24 kDa, pI= 5.5) is contaminated with two other proteins, one witha similar molecular mass and a pI of 7.0 while the other has a molecular mass of 100 kDa and a pIof 5.4. Suggest how the contaminated enzyme can be purified.arrow_forward
- The net charge on the most prevalent form of bisphosphoglycerate in blood is what?arrow_forwardWhat is the role of imidazole in Ni-Affinity Chromatography?arrow_forwardFor the following reaction 3 experiments have been run and the data collected is in the following table @ 35 degrees Celsius 2 NO2F(g) ---> 2 NO2(g) + F2(g) Experiment [NO2F], M Rates, M/s 1 0.263 0.168 2 0.349 0.223 3 0.421 0.269 a) How long will it take for a 65% NO2F solution to become a 31% NO2F solution @35 degrees Celsius?(Hint: Use mass ratios and assume ~1g/ml for density of solutions to get you started) b) It has been determined that at 75 degrees Celsius the rate constant is 1.046 s-1. Calculate the activation energy for the decomposition of NO2F. [Hint: ]ln?1?2=?a?(1?2―1?1) c) What is the half-life of a 35% solution of NO2F @ 35 degrees Celsius?arrow_forward
- Consider the reaction A + 2B ----> C. If the molar mass of C is twice the molar mass of A, what mass of C is produced by the complete reaction of 10.0 g A?(a) 10.0 g(b) 30.0 g(c) 60.0 garrow_forwardIf the average molar mass of a sample of soybean oil is 1500 g/mol, how many grams of NaOH are needed to saponify 5.0 g of the oil?arrow_forwardIf you measured the rate of reaction at 20°C to be 1.11 x 10-5 M/s when using 0.080 M I1- and 0.040 M S2O82-. Approximately how long will the reaction take if you were to increase the temperature to 30 °C?arrow_forward
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON





