
When boron trifluoride reacts with ammonia, the following reaction occurs:
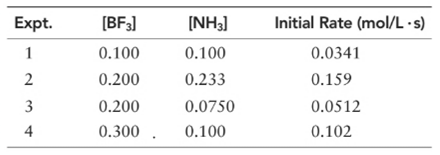
(a) What is the order of the reaction with respect to BF3, NH3, and overall?
(b) Write the rate expression for the reaction.
(c) Calculate k for the reaction.
(d) When
(a)
Interpretation:
To determine the order of reaction with respect to BF3, NH3 and overall for the following reaction:
Concept introduction:
Rate of a chemical reaction: It tells us about the speed at which the reactants are converted into products.
Mathematically, rate of reaction is directly proportional to the product of concentration of each reactant raised to the power equal to their respective stoichiometric coefficients.
Let’s say we have a reaction:
Answer to Problem 25QAP
Order of given reaction
With respect to BF3 =1
With respect to NF3 =1
Overall = 2.
Explanation of Solution
Given information:
Here the chemical reaction is:

Let’s assume the reaction to be ‘t’ order with respect to BF3 and ‘y’ order with respect to NH3.
Then, rate law for experiment 1 in above reaction will be:
And, rate law for experiment 4 in above reaction will be;
Divide (1) by (2) to get value of ‘t’.
Now writing rate law for experiment 2 in above reaction will be;
And, rate law for experiment 4 in above reaction will be;
Divide (3) by (4) to get value of ‘y’.
Thus, order with respect to NH3 is 1.
And the order of reaction will be:
Thus, overall order of reaction is 2.
(b)
Interpretation:
To write the rate expression for the given reaction.
Concept introduction:
Rate of a chemical reaction: It tells us about the speed at which the reactants are converted into products.
Mathematically, rate of reaction is directly proportional to the product of concentration of each reactant raised to the power equal to their respective stoichiometric coefficients.
Let’s say we have a reaction:
Answer to Problem 25QAP
Rate law expression for the given reaction will be;
Explanation of Solution
Here the chemical reaction is:
Order of reaction with respect to BF3 = 1
Order of reaction with respect to NH3 = 1
Let the rate constant be ‘k’.
Then, rate law expression for above reaction will be;
(c)
Interpretation:
To determine the rate constant for the given reaction.
Concept introduction:
Rate of a chemical reaction: It tells us about the speed at which the reactants are converted into products.
Mathematically, rate of reaction is directly proportional to the product of concentration of each reactant raised to the power equal to their respective stoichiometric coefficients.
Let’s say we have a reaction:
Answer to Problem 25QAP
Rate constant for given reaction is
Explanation of Solution
Here the chemical reaction is:
Rate law expression for above reaction:
Plugging values in rate law expression for experiment 1 as:
Hence, the rate constant is
(d)
Interpretation:
To determine the rate of reaction at given concentration of reactants.
Concept introduction:
Rate of a chemical reaction: It tells us about the speed at which the reactants are converted into products.
Mathematically, rate of reaction is directly proportional to the product of concentration of each reactant raised to the power equal to their respective stoichiometric coefficients.
Let’s say we have a reaction:
Answer to Problem 25QAP
Rate of reaction for given reaction at given conditions is
Explanation of Solution
Here the chemical reaction is:
Rate law expression for above reaction:
Here we have:
[BF3 ]= 0.533 M
[NH3 ] = 0.300 M
Rate constant = 3.41 L/mol.s
Plugging values in rate law as:
Hence, the rate of reaction is
Want to see more full solutions like this?
Chapter 11 Solutions
Chemistry: Principles and Reactions
- Diethylhydrazine reacts with iodine according to the following equation: Â (C2H5)2(NH)2(l)+I2(aq)(C2H5)2N2+2HI(aq)The rate of the reaction is followed by monitoring the disappearance of the purple color due to iodine. The following data are obtained at a certain temperature. (a) What is the order of the reaction with respect to diethylhydrazine, iodine, and overall? (b) Write the rate expression of the reaction. (c) Calculate k for the reaction. (d) What must [(C2H5)2] be so that the rate of the reaction is 5.00104mol/Lh when [ I2 ]=0.500M?arrow_forwardFor a reaction involving the decomposition of Z at a certain temperature, the following data are obtained: (a) What is the order of the reaction? (b) Write the rate expression for the decomposition of Z. (c) Calculate k for the decomposition at that temperature.arrow_forwardAt 573 K, gaseous NO2(g) decomposes, forming NO(g) and O2(g). If a vessel containing NO2(g) has an initial concentration of 1.9 102 mol/L, how long will it take for 75% of the NO2(g) to decompose? The decomposition of NO2(g) is second-order in the reactant and the rate constant for this reaction, at 573 K, is 1.1 L/mol s.arrow_forward
- The decomposition of iodoethane in the gas phase proceeds according to the following equation: C2H5I(g)C2H4(g)+HI(g) At 660. K, k = 7.2 104 sl; at 720. K, k = 1.7 102 sl. What is the value of the rate constant for this first-order decomposition at 325C? If the initial pressure of iodoethane is 894 torr at 245C, what is the pressure of iodoethane after three half-lives?arrow_forwardUnder certain conditions the decomposition of ammonia on a metal surface gives the following data: [NH3] (M) 1.0103 2.0103 3.0103 Rate (moI/L/h1) 1.5106 1.5106 1.5106 Determine the rate equation, the rate constant, and the overall order for this reaction.arrow_forwardSucrose, a sugar, decomposes in acid solution to give glucose and fructose. The reaction is first-order in sucrose, and the rate constant at 25 C is k = 0.21 h1. If the initial concentration of sucrose is 0.010 mol/L, what is its concentration after 5.0 h?arrow_forward
- For a reaction involving the decomposition of a hypothetical substance Y, these data are obtained: Determine the order of the reaction. Write the rate law for the decomposition of Y. Calculate k for the experiment above.arrow_forwardWhen nitrogen dioxide reacts with carbon monoxide, the following reaction occurs. Â NO2(g)+CO(g)NO(g)+CO2(g)The following data are obtained at a certain temperature: (a) What is the order of the reaction with respect to NO2, CO, and overall? (b) Write the rate expression of the reaction. (c) Calculate k for the reaction. (d) When [ NO2 ]=0.421Mand [ CO ]=0.816M, what is the rate of the reaction at the temperature of the experiments?arrow_forwardThe half-life of tritium, 3H, is 12.26 years. Tritium is the radioactive isotope of hydrogen. (a) What is the rate constant for the radioactive decay of tritium, in y1 and s1? (b) What percentage of the original tritium is left after 61.3 years?arrow_forward
- The reaction for the Haber process, the industrial production of ammonia, is N2(g)+3H2(g)2NH3(g) Assume that under certain laboratory conditions ammonia is produced at the rate of 6.29 ×10-5 molL-1s-1. At what rate is nitrogen consumed? At what rate is hydrogen consumed?arrow_forwardThe following rate constants were obtained in an experiment in which the decomposition of gaseous N2O; was studied as a function of temperature. The products were NO, and NO,. Temperature (K) 3.5 x 10_i 298 2.2 x 10"4 308 6.8 X IO-4 318 3.1 x 10 1 328 Determine Etfor this reaction in kj/mol.arrow_forwardExpress the rate of the reaction 2N2O(g)2N2(g)+O2(g) in terms of (b) [ N2O ] (a) [ O2 ]arrow_forward
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning





