
Organic Chemistry
9th Edition
ISBN: 9781305080485
Author: John E. McMurry
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Chapter 1.10, Problem 14P
Interpretation Introduction
a) The oxygen atom in dimethyl ether, CH3-O-CH3
Interpretation:
The number of nonbonding lone pair of electrons present on oxygen atom in dimethyl ether is to be identified. Further its expected geometry is to be stated.Concept introduction:
The electrons present in the valence shell of an atom that are not involved in bonding with other atoms are called nonbonding or lone pair of electrons. In a molecule, if an atom has only single electrons in all hybridized orbitals then the bonds formed by these orbitals will be equivalent in all respects. The molecule will thus have a regular structure. But if the atom contains single electron as well as unshared pairs of electrons in the hybridized orbitals, the orbitals with unshared pair of electrons will tend to occupy as much space as those orbitals involved in bonding. The bond angles will be slightly different from the expected bond angle and hence the molecule will not have a regular geometry.To determine:
The number of nonbonding lone pair of electrons present on oxygen atom in dimethyl ether and its expected geometry.Interpretation Introduction
b) The nitrogen atom in trimethylamine, CH3-N- [CH3]2
Interpretation:
The number of nonbonding lone pair of electrons present on nitrogen atom in trimethylamine, is to be identified. Further its expected geometry is to be stated.Concept introduction:
The electrons present in the valence shell of an atom that are not involved in bonding with other atoms are called nonbonding or lone pair of electrons. In a molecule, if an atom has only single electrons in all hybridized orbitals then the bonds formed by these orbitals will be equivalent in all respects. The molecule will thus have a regular structure. But if the atom contains single electron as well as unshared pairs of electrons in the hybridized orbitals, the orbitals with unshared pair of electrons will tend to occupy as much space as those orbitals involved in bonding. The bond angles will be slightly different from the expected bond angle and hence the molecule will not have a regular geometry.To determine:
The number of nonbonding lone pair of electrons present on nitrogen atom in trimethylamine and its expected geometry.Interpretation Introduction
c) The phosphorus atom in phosphine, PH3
Interpretation:
The number of nonbonding lone pair of electrons present on phosphorus atom in phosphine is to be identified. Further the expected geometry of phosphorus atom in phosphine is to be stated.Concept introduction:
The electrons present in the valence shell of an atom that are not involved in bonding with other atoms are called nonbonding or lone pair of electrons. In a molecule, if an atom has only single electrons in all hybridized orbitals then the bonds formed by these orbitals will be equivalent in all respects. The molecule will thus have a regular structure. But if the atom contains single electron as well as unshared pairs of electrons in the hybridized orbitals, the orbitals with unshared pair of electrons will tend to occupy as much space as those orbitals involved in bonding. The bond angles will be slightly different from the expected bond angle and hence the geometry of the molecule will be pyramidal.To determine:
The number of nonbonding lone pair of electrons present on phosphorus atom in phosphine and its expected geometry.Answer:
The phosphorus atom in phosphine has one lone pair of electrons. The phosphorus atom is in sp3 hybridized state with one orbital occupied by lone pairs of electrons. Hence the geometry will be pyramid.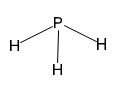
Explanation:
Phosphorus atom has five electrons in its valence shell. It has formed three single bonds with three hydrogen atoms in phosphine. Therefore one lone pair of electrons remains on phosphorus atom. In phosphine the phosphorus atom is in sp3 hybridized state. Three of the sp3 hybrid orbitals containing single electron are utilized for forming three P-H sigma bonds. The fourth sp3 hybrid orbital accommodates the lone pair of electrons and it occupy as much space as a P-H bond does. The H-P-H bond angles deviate slightly from the normal tetrahedral angle. Hence the shape is pyramidal.Conclusion:
The phosphorus atom in phosphine has one lone pair of electrons. The phosphorus atom is in sp3 hybridized state with one orbital occupied by lone pairs of electrons. Hence the structure will be pyramidal.
Interpretation Introduction
d) The sulfur atom in the amino acid methionine
Interpretation:
The number of nonbonding lone pair of electrons present on sulfur atom in the amino acid methionine is to be identified and to state its expected geometry.Concept introduction:
The electrons present in the valence shell of an atom that are not involved in bonding with other atoms are called nonbonding or lone pair of electrons. In a molecule, if an atom has only single electrons in all hybridized orbitals then the bonds formed by these orbitals will be equivalent in all respects. The molecule will thus have a regular structure. But if the atom contains single electron as well as unshared pairs of electrons in the hybridized orbitals, the orbitals with unshared pair of electrons will tend to occupy as much space as those orbitals involved in bonding. The bond angles will be slightly different from the expected bond angle.To determine:
The number of nonbonding lone pair of electrons present on sulfur atom in the amino acid methionine and its expected geometry.Expert Solution & Answer
Trending nowThis is a popular solution!

Chapter 1 Solutions
Organic Chemistry
Ch. 1.3 - Give the ground-state electron configuration for...Ch. 1.3 - How many electrons does each of the following...Ch. 1.4 - Prob. 3PCh. 1.4 - Convert the following representation of ethane,...Ch. 1.4 - What are likely formulas for the following...Ch. 1.4 - Prob. 6PCh. 1.4 - Prob. 7PCh. 1.7 - Draw a line-bond structure for propane, CH3CH2CH3....Ch. 1.7 - Convert the following molecular model of hexane, a...Ch. 1.8 - Draw a line-bond structure for propene, CH3CH=CH2....
Ch. 1.8 - Draw a line-bond structure for 1, 3-butadiene,...Ch. 1.8 - Following is a molecular model of aspirin...Ch. 1.9 - Draw a line-bond structure for propyne, CH3C≡CH....Ch. 1.10 - Prob. 14PCh. 1.12 - Prob. 15PCh. 1.12 - Prob. 16PCh. 1.12 - The following molecular model is a representation...Ch. 1.SE - Convert each of the following molecular models...Ch. 1.SE - The following model is a representation of citric...Ch. 1.SE - The following model is a representation of...Ch. 1.SE - The following model is a representation of...Ch. 1.SE - How many valence electrons does each of the...Ch. 1.SE - Give the ground-state electron configuration for...Ch. 1.SE - Prob. 24APCh. 1.SE - Prob. 25APCh. 1.SE - Draw an electron-dot structure for acetonitrile,...Ch. 1.SE - Draw a line-bond structure for vinyl chloride,...Ch. 1.SE - Fill in any nonbonding valence electrons that are...Ch. 1.SE - Convert the following line-bond structures into...Ch. 1.SE - Convert the following molecular formulas into...Ch. 1.SE - Prob. 31APCh. 1.SE - Oxaloacetic acid, an important intermediate in...Ch. 1.SE - Prob. 33APCh. 1.SE - Potassium methoxide, KOCH3, contains both covalent...Ch. 1.SE - What is the hybridization of each carbon atom in...Ch. 1.SE - Prob. 36APCh. 1.SE - Prob. 37APCh. 1.SE - What bond angles do you expect for each of the...Ch. 1.SE - Propose structures for molecules that meet the...Ch. 1.SE - What kind of hybridization do you expect for each...Ch. 1.SE - Pyridoxal phosphate, a close relative of vitamin...Ch. 1.SE - Prob. 42APCh. 1.SE - Prob. 43APCh. 1.SE - Quetiapine, marketed as Seroquel, is a heavily...Ch. 1.SE - Tell the number of hydrogens bonded to each carbon...Ch. 1.SE - Why do you suppose no one has ever been able to...Ch. 1.SE - Allene, H2C=C=CH2, is somewhat unusual in that it...Ch. 1.SE - Allene (see Problem 1-47) is structurally related...Ch. 1.SE - Complete the electron-dot structure of caffeine,...Ch. 1.SE - Most stable organic species have tetravalent...Ch. 1.SE - A carbanion is a species that contains a...Ch. 1.SE - Divalent carbon species called carbenes are...Ch. 1.SE - There are two different substances with the...Ch. 1.SE - There are two different substances with the...Ch. 1.SE - There are two different substances with the...Ch. 1.SE - Prob. 56APCh. 1.SE - Among the most common over-the-counter drugs you...
Knowledge Booster
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY