
Concept explainers
The process that causes the greatest amount of exergy destruction.
Answer to Problem 117RP
The process that causes the greatest amount of exergy destruction is
Explanation of Solution
Show the T-s diagram for refrigeration system as in Figure (1).
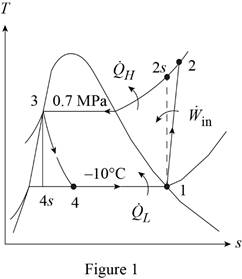
From Figure (1), write the specific enthalpy at state 3 is equal to state 4 due to throttling process.
Here, specific enthalpy at state 3 and 4 is
Express specific enthalpy at state 2 using compressor efficiency.
Here, specific enthalpy at state 2, 1 and 2s is
Express temperature at state 3.
Here, saturation temperature at pressure of
Express quality at state 4.
Here, specific enthalpy at saturation liquid and temperature of
Express specific entropy at state 4.
Here, specific entropy at saturation liquid and temperature of
Express heat rejected in the evaporator.
Here, specific enthalpy at state 4 is
Express heat added in the condenser.
Express the exergy destruction during process 1-2.
Here, surrounding temperature is
Express the exergy destruction during process 2-3.
Here, specific entropy at state 3 is
Express the exergy destruction during process 3-4.
Here, specific entropy at state 4 is
Express the exergy destruction during process 4-1.
Here, freezing temperature of water is
Conclusion:
Refer Table A-11, “saturated refrigerant-134a-temperature table”, and write the properties corresponding to initial temperature
Here, specific entropy at state 1 is
Perform unit conversion of pressure at state 2 from
Refer Table A-13, “superheated refrigerant 134a”, and write the specific enthalpy at state 2s corresponding to pressure at state 2 of
Write the formula of interpolation method of two variables.
Here, the variables denote by x and y is specific entropy at state 2 and specific enthalpy at state 2s respectively.
Show the specific enthalpy at state 2s corresponding to specific entropy as in Table (1).
|
Specific entropy at state 2 |
Specific enthalpy at state 2s |
| 0.9314 | 268.47 |
| 0.9378 | |
| 0.9642 | 278.59 |
Substitute
Thus, the specific enthalpy at state 2s is,
Refer Table A-12, “saturated refrigerant-134a-pressure table”, and write the saturated temperature corresponding to pressure of
Substitute
Refer Table A-11, “saturated refrigerant-134a-temperature table”, and write the specific enthalpy and entropy at state 3 corresponding to temperature at state 3
Here, specific enthalpy and entropy at saturated liquid is
Substitute
Substitute
Refer Table A-11, “saturated refrigerant-134a-temperature table”, and write the properties corresponding to final temperature
Substitute
Substitute
Refer Table A-13, “superheated refrigerant 134a”, and write the specific entropy at state 2 corresponding to pressure at state 2 of
Show the specific entropy at state 2 corresponding to specific enthalpy as in Table (2).
|
Specific enthalpy at state 1 |
Specific entropy at state 2 |
| 268.47 | 0.9314 |
| 275.02 | |
| 278.59 | 0.9642 |
Use excels and tabulates the values from Table (2) in Equation (XII) to get,
Substitute
Substitute
Perform unit conversion of temperature from
Substitute
Substitute
Substitute
Take the freezing temperature of water as,
Substitute
Hence, the process that causes the greatest amount of exergy destruction is
Want to see more full solutions like this?
Chapter 11 Solutions
THERMODYNAMICS
- In a steam power plant, saturated vapor enters the turbine at 7.5 MPa, while having a condenser pressure at 7.5 kPa. Determine the (a )network per unit mass of steam flow: (b) heat transfer to the stream passing through the boiler and (c) thermal efficiencyarrow_forwardConsider a 210-MW steam power plant that operates on a simple ideal Rankine cycle. Steam enters the turbine at 10 MPa and 500C and is cooled in the condenser at a pressure of 10 kPa. Determine the mass flow rate of the stream (kg/s). (Use 2 decimal places for the final answer.)arrow_forwardThe enthalpy of steam entering the turbine inlet is 2750 kJ/kg and leaving turbine at 1750 kJ/kg. The enthalpy of saturated liquid is 190 kJ/ kg and enthalpy of dry saturated steam 2600 kJ/kg corresponding to the condenser pressure. The quality of steam at the exit of turbine isarrow_forward
- Consider a steam power plant with a reheat Rankine cycle. The steam enters the high pressure turbine at 16 MPa and 550 ° C, and condenses in the condenser at 10 kPa. If the moisture content of the steam at the outlet of the low pressure turbine should not exceed 5%, determine: a) The pressure to which the steam should be reheated. b) The thermal efficiency of the cyclearrow_forwardIn a steam power plant, the condenser pressure is 10 kPa. The turbine and pump isentropic efficiencies are both 85 %. Draw the schematic and T-S diagrams. Label the points by setting point 1 at the condenser outlet, point 2 at the pump outlet, point 3 at the boiler outlet, and point 4 at the turbine outlet. Use the label 2a and 4a for the points due to the isentropic efficiency of the pump and turbine, respectively. Use 2 decimal places for the enthalpy and other energies in solving and for the final answers. For the steam quality (x) and entropy (s), use 4 decimal places in solving. For the specific volume, use 6 decimal places. The pressure and the temperature of steam that enters the turbine are 4.5 MPa and 800 oC Determine the following: (INPUT YOUR ANSWERS ON THE BLANK SPACES PROVIDED.) Enthalpy at point 1 in kJ/kg = Enthalpy at point 2 in kJ/kg = Enthalpy at point 3 in kJ/kg = Enthalpy at point 4 in kJ/kg = Actual Enthalpy at point 2a in kJ/kg = Actual Enthalpy at point…arrow_forwardRefrigerant 134a is used as the working fluid in a refrigerator, and it operates in an ideal vapor compression refrigeration cycle, if the refrigerant enters the evaporator at -4 ° C and enters the condenser at 1200 kPa. If the mass flow of the refrigerant is 180 kg / h, determine a) the Ts and Ph diagrams of the cycle with all the information b) the rate of heat removal from the refrigerated space and the power input to the compressor, and c) the COP Of fridgearrow_forward
- As the refrigerant as shown in the figure, R134a fluid is used in a commercial refrigerator. The refrigerant leaves the evaporator at a temperature of -33.5°C (239.65 K) and a pressure of 60 kPa. It is then compressed to a pressure of 1.2 MPa and a temperature of 64°C (337.15 K). In the water-cooled condenser, the refrigerant is cooled to 41°C (314.15 K). Water enters the condenser at 18°C and leaves at 26°C. In this context; *Calculate the COP value and the maximum (maximum) COP value of the refrigerator.arrow_forwardAs the refrigerant as shown in the figure, R134a fluid is used in a commercial refrigerator. The refrigerant leaves the evaporator at a temperature of -33.5°C (239.65 K) and a pressure of 60 kPa. It is then compressed to a pressure of 1.2 MPa and a temperature of 64°C (337.15 K). In the water-cooled condenser, the refrigerant is cooled to 41°C (314.15 K). Water enters the condenser at 18°C and leaves at 26°C. In this context; *Calculate the COP value and the maximum (maximum) COP value of the refrigerator. (I ask you to write with the keyboard so that I can understand the answers. Thank you)arrow_forwardAs the refrigerant as shown in the figure, R134a fluid is used in a commercial refrigerator. The refrigerant leaves the evaporator at a temperature of -33.5°C (239.65 K) and a pressure of 60 kPa. It is then compressed to a pressure of 1.2 MPa and a temperature of 64°C (337.15 K). In the water-cooled condenser, the refrigerant is cooled to 41°C (314.15 K). Water enters the condenser at 18°C and leaves at 26°C. In this context; *Calculate the degree of dryness (quality) of the refrigerant at the evaporator inlet [%]. (I ask you to write with the keyboard so that I can understand the answers. Thank you)arrow_forward
- As the refrigerant as shown in the figure, R134a fluid is used in a commercial refrigerator. The refrigerant leaves the evaporator at a temperature of -33.5°C (239.65 K) and a pressure of 60 kPa. It is then compressed to a pressure of 1.2 MPa and a temperature of 64°C (337.15 K). In the water-cooled condenser, the refrigerant is cooled to 41°C (314.15 K). Water enters the condenser at 18°C and leaves at 26°C. In this context; *Calculate the degree of dryness (quality) of the refrigerant at the evaporator inlet [%].arrow_forwardAs the refrigerant as shown in the figure, R134a fluid is used in a commercial refrigerator. The refrigerant leaves the evaporator at a temperature of -33.5°C (239.65 K) and a pressure of 60 kPa. It is then compressed to a pressure of 1.2 MPa and a temperature of 64°C (337.15 K). In the water-cooled condenser, the refrigerant is cooled to 41°C (314.15 K). Water enters the condenser at 18°C and leaves at 26°C. In this context; *Set the cooling load in [kW].arrow_forwardA supercritical power plant generates steam at 25 Mpa and 560C. The condenser pressure is 7.0 kpa. Determine the exit quality of steam if it expands through a turbine in this power plant.arrow_forward
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY





