
ORGANIC CHEMISTRY
4th Edition
ISBN: 9781119745105
Author: Klein
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 11.3, Problem 6ATS
Interpretation Introduction
Interpretation: A proper synthesis for the following transformation is to be determined.
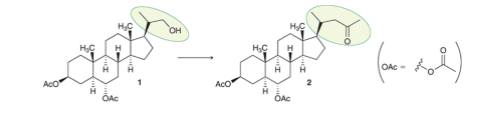
Concept introduction: The transformation of an alcoholic group to a
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
A carbamate can be prepared by treating an isocyanate
with an alcohol, as shown here. This type of reaction is
used to synthesize polyurethanes- polymers that have
a wide variety of industrial applications, such as surface
sealants, high-performance adhesives, and synthetic
fibers. Propose a mechanism for this transformation.
R'—ОН
R.
`N=C=0
R.
OR'
An isocyanate
A carbamate
(Substituted urethane)
O=C
C10T03Q1068
Give the major product of the following reaction.
HBr
((CH3)3CO)2
HOOCH
H CH₂NH2
Tranexamic acid, a drug useful against blood clotting, is prepared commercially from p-methylbenzonitrile. Following is one of the steps in its synthesis, draw the
structure of the product of this step.
COOH
0
CH₂Br
989
་་་
1. excess NH3.
2. H3O+
?
n
?
Previous
Next
Chapter 11 Solutions
ORGANIC CHEMISTRY
Ch. 11.1 - Prob. 1CCCh. 11.1 - Prob. 2CCCh. 11.2 - Prob. 1LTSCh. 11.2 - Prob. 3PTSCh. 11.3 - Prob. 2LTSCh. 11.3 - Prob. 6ATSCh. 11.4 - Prob. 3LTSCh. 11.4 - Prob. 7PTSCh. 11.4 - Prob. 8ATSCh. 11.5 - Prob. 4LTS
Ch. 11.5 - Propose an efficient synthesis for each of the...Ch. 11.5 - Prob. 10ATSCh. 11 - Prob. 11PPCh. 11 - Prob. 12PPCh. 11 - Prob. 13PPCh. 11 - Prob. 14PPCh. 11 - Prob. 15PPCh. 11 - Prob. 16PPCh. 11 - Prob. 17PPCh. 11 - Prob. 18PPCh. 11 - Prob. 19PPCh. 11 - Prob. 20PPCh. 11 - Prob. 21PPCh. 11 - Prob. 22PPCh. 11 - Prob. 23PPCh. 11 - Prob. 24PPCh. 11 - Prob. 25PPCh. 11 - Prob. 26ASPCh. 11 - Prob. 27ASPCh. 11 - Prob. 28ASPCh. 11 - Prob. 29ASPCh. 11 - Prob. 30ASPCh. 11 - Prob. 31ASPCh. 11 - Prob. 32ASPCh. 11 - Prob. 33IPCh. 11 - Prob. 34IPCh. 11 - Prob. 35IPCh. 11 - Prob. 36IPCh. 11 - Prob. 37IPCh. 11 - Prob. 38IPCh. 11 - Prob. 39IPCh. 11 - Prob. 42CPCh. 11 - The compound Z-3-hexenyl acetate is one of several...Ch. 11 - When a consumer purchases a tomato, smell is one...Ch. 11 - Prob. 45CPCh. 11 - Prob. 46CP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Which of the following is an effective way to complete the synthes below. - © LĨNH, 2) H₂O* 1) HNO₂H₂50 2) H₂/Ethanol (can't reduce ring) O DILDA 2) NANO 1) NaOH 2)CHÍNH)arrow_forwardWhen ethoxybenzene is treated with a mixture of nitric acid and sulfuric acid, two products are obtained, each of which has the molecular formula C8H9NO3. For the mechanism, draw the curved arrows as needed. Include lone pairs and charges in your answer. Do not draw out any hydrogen explicitly in your products. Do not use abbreviations such as Me or Ph.arrow_forwardprovide the hydrotreating for the organic compoud C9H7N in a more detailed way? regarding which bond will break first and how many steps the reactant will go through to reach the product shown.arrow_forward
- Acyl transfer (nucleophilic substitution at carbonyl) reactions proceed in two stages via a "tetrahedral intermediate." Draw the tetrahedral intermediate as it is first formed in the following reaction. You do not have to consider stereochemistry. Include all valence lone pairs in your answer. Do not include counter-ions, e.g., Na+, I-, in your answer. In cases where there is more than one answer, just draw one.arrow_forwardCan you please help show how to use the given reactant in order to synthesize this molecule?arrow_forwardQ17. Compound 1 can undergo an intramolecular reaction to give cyclic product 2. Using curly arrows, show the mechanism of this reaction – note that the mechanism involves more than one elemental step - and draw the structure of 2; chemical formula is provided as guidance. H2N. C5H9NO CH;OH 2arrow_forward
- How do we synthesize compound 2 from benzene?arrow_forwardWhen 5-hexen-3-one is reacted with slightly less H2 than substrate, along with Pt, Pd or Ni metal, the major substrate product will contain the following functional groups: alkene & alcohol. alkene & ketone, since no reaction occurs under these conditions. alkene only. ketone only. alcohol only.arrow_forwardAlkenes can be converted into alcohols by acid-catalyze addition of water. Predict the major alcohol product from this alkene below.arrow_forward
- d) Synthesize the following compound starting from benzene. NO₂arrow_forwardMechanism 2. Provide the complete mechanism for the reactions below. You must include appropriate arrows, intermediates, and formal charges. The ChemDraw template of this document is available on Carmen. OH H2SO4 (cat) THF OHarrow_forwardComplete the mechanism of hydration of alkene below. Please follow curved arrows to predict the resulting structures in each step Step 1 of hydration of 2-methyl-1-propene (Note that the most stable carbocation is formed here) Step 2 of hydration of 2-methyl-1-propene(Note that the most stable carbocation is formed here) Step 3 of hydration of 2-methyl-1-propenearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
How to Design a Total Synthesis; Author: Chemistry Unleashed;https://www.youtube.com/watch?v=9jRfAJJO7mM;License: Standard YouTube License, CC-BY