
Concept explainers
(a)
Interpretation:
The resultant product obtained from the metathesis of the alkene
Concept introduction:
Alkene Metathesis Or olefin metathesis
This breaks the double bond of an alkene and then rejoins the fragments. When the fragments are joined, the new double bond is formed between two
Terminal alkene gives the best yields of a single alkene product in metathesis because one of the products is ethane, which is equally removed from the reaction mixture, thus shifting the equilibrium in favor of the other new alkene product.
(b)
Interpretation:
The resultant product obtained from the metathesis of the following alkene should be identified.
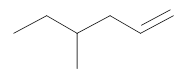
Concept introduction:
Alkene Metathesis Or olefin metathesis
This breaks the double bond of an alkene and then rejoins the fragments. When the fragments are joined, the new double bond is formed between two
Terminal alkene gives the best yields of a single alkene product in metathesis because one of the products is ethane, which is equally removed from the reaction mixture, thus shifting the equilibrium in favor of the other new alkene product.
(c)
Interpretation:
The resultant product obtained from the metathesis of the following alkene should be identified.
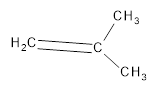
Concept introduction:
Alkene Metathesis Or (olefin metathesis)
This breaks the double bond of an alkene and then rejoins the fragments. When the fragments are joined, the new double bond is formed between two
Terminal alkene gives the best yields of a single alkene product in metathesis because one of the products is ethane, which is equally removed from the reaction mixture, thus shifting the equilibrium in favor of the other new alkene product.
(d)
Interpretation:
The resultant product obtained from the metathesis of the following alkene should be identified.
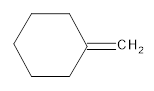
Concept introduction:
Alkene Metathesis Or (olefin metathesis)
This breaks the double bond of an alkene and then rejoins the fragments. When the fragments are joined, the new double bond is formed between two
Terminal alkene gives the best yields of a single alkene product in metathesis because one of the products is ethane, which is equally removed from the reaction mixture, thus shifting the equilibrium in favor of the other new alkene product.
Trending nowThis is a popular solution!

Chapter 11 Solutions
MODIFIED MASTERING CHEMISTRY W/ ETEXT
- yredict the NAME, STRUCTURE and STEREOCHEMISTRY of the BEST ORGANIC REACTANT or the MAJOR ORGANIC product for the following reactions:arrow_forwardStarting with bromocyclohexane, how can each of the following compounds be prepared?arrow_forwardDraw the alkene that would react with the reagent given to account for the product formed. ? + H₂O H₂SO4 CH3 CH3 CHCCH3 OH CH3 • You do not have to consider stereochemistry. • You do not have to explicitly draw H atoms. • In cases where there is more than one answer, just draw one. Sn [F ChemDoodlearrow_forward
- Zaitsev's Rule refers to: which alkene is favored in a product mixture O how resonance contributors stabilize cations/anions/radicals O the stereochemistry of reaction intermediates/transition states O the stability of carbocation intermediatesarrow_forwardWhich of the following alkenes has the lowest heat of hydrogenation?arrow_forwardWhich alkene product would you expect to be the major product under kinetic conditions? Under thermodynamic conditions? The given pictures are the reactions in which we have to determine which alkene product we expect would be the major product under kinetic and thermodynamic conditions.arrow_forward
- What reagents would you use to prepare each of the following from 3-hexene? a. b.CH3CH2CH2CH2CH2CH3 c. d.arrow_forwardМСРВА Alkenes are oxidized to give epoxides on treatment with a peroxyacid, RCO3H, such as metachloroperoxybenzoic acid (MCPBA). Peroxyacids transfer an oxygen atom to the alkene with syn stereochemistry, i.e. both C-O bonds form on the same face of the double bond, through a single step mechanism without intermediates. The oxygen atom farthest from the carbonyl group is the one transferred. Draw curved arrows to show the movement of electrons in this step of the mechanism. Arrow-pushing Instructions H .CI .CI HOarrow_forward.Which one of the following molecules can react as electrophile in reactions with alkenes? CH3OH NaCI HCI KCN NaCI O HCI O KCN O CH3OHarrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
