
Essential Organic Chemistry (3rd Edition)
3rd Edition
ISBN: 9780321937711
Author: Paula Yurkanis Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Question
Chapter 11.8, Problem 17P
Interpretation Introduction
Interpretation:
To write the mechanism for the acid-catalyzed transesterification of ethyl acetate and methanol.
Concept introduction:
Transesterification is the process of formation of a new ester molecule from the reaction of alcohol and an ester. This is like hydrolysis of ester but here nucleophile is alcohol molecule instead of
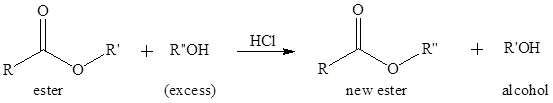
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
write the mechanism for the acid-catalyzed transesterification reaction of methyl acetate with ethanol.
Write the mechanism for the acid-catalyzed reaction of tert-butyl acetate with methanol.
Write a mechanism for the acid-catalyzed esterification of acetic acid with pentanol
Chapter 11 Solutions
Essential Organic Chemistry (3rd Edition)
Ch. 11.1 - The aromas of many flowers and fruits are due to...Ch. 11.1 - Name the following compounds:Ch. 11.1 - Prob. 3PCh. 11.2 - Prob. 4PCh. 11.2 - Prob. 5PCh. 11.4 - a. What is the product of the reaction of acetyl...Ch. 11.4 - Prob. 7PCh. 11.5 - Using the pKa values listed in Table 11.1, predict...Ch. 11.6 - Starting with acetyl chloride, what neutral...Ch. 11.6 - Prob. 10P
Ch. 11.7 - Prob. 11PCh. 11.8 - Prob. 13PCh. 11.8 - Using the mechanism for the acidcatalyzed...Ch. 11.8 - Prob. 15PCh. 11.8 - Prob. 16PCh. 11.8 - Prob. 17PCh. 11.9 - Prob. 18PCh. 11.10 - Show how each of the following esters could be...Ch. 11.11 - Which of the following reactions would lead to the...Ch. 11.12 - Prob. 22PCh. 11.12 - Prob. 23PCh. 11.13 - Prob. 24PCh. 11.13 - Prob. 25PCh. 11.14 - Prob. 26PCh. 11.14 - Prob. 27PCh. 11.14 - Prob. 28PCh. 11.15 - Prob. 29PCh. 11.15 - How would you synthesize the following compounds...Ch. 11 - Write a structure for each of the following a. N,N...Ch. 11 - Prob. 32PCh. 11 - Which ester is more reactive, methyl acetate or...Ch. 11 - What products would be formed from the reaction of...Ch. 11 - What products would be obtained from the following...Ch. 11 - Prob. 36PCh. 11 - a. Which compound would you expect to have a...Ch. 11 - a. List the following esters in order of...Ch. 11 - D. N. Kursanov, a Russian chemist, proved that the...Ch. 11 - Prob. 40PCh. 11 - Using an alcohol for one method and an alkyl...Ch. 11 - Prob. 42PCh. 11 - Prob. 44PCh. 11 - Prob. 45PCh. 11 - Prob. 46PCh. 11 - Prob. 47PCh. 11 - Prob. 48PCh. 11 - Prob. 49PCh. 11 - Show how the following compounds could be prepared...Ch. 11 - Prob. 51PCh. 11 - Prob. 52PCh. 11 - Prob. 53P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Write the mechanism of the reaction of phenylmethyl ketone with Br2 in the presence of NaOH.arrow_forwardWrite a mechanism for the enolization of a ketone. Show how acids and bases can act as catalysists of this reaction.arrow_forwardWrite the mechanism for the formation of aniline with 1-naphthol.arrow_forward
- Write the complete mechanism for the acid-catalyzed hydrolysis of acetonitrile to ethanoic acidarrow_forwardWrite a mechanism for the acid-catalyzed esterification of acetic acid with isopentyl alcohol.arrow_forwardUsing the principles for writing mechanisms and the four common mechanistic steps, write mechanisms showing all electron flow arrows for the following reaction : (a) Hydrolysis of N,N-dimethylacetamide in acidic water.arrow_forward
- Write the complete mechanism for the hydroxide ion catalyze reaction of cyclohexanone and 1-methylcyclohexanecarbaldehydearrow_forwardWrite a detailed mechanism for oxidative dehydrogenation of isobutyric acid to methacrylic acid.arrow_forwardis there a more intricate mechanism of the acid-catalyzed esterification of acetic acid with pentanol in the presence of the acid catalyst sulfuric acidarrow_forward
- Write a complete reaction mechanism for this base-catalyzed transesterification reaction. Rather than starting with a complete oil molecule, give the mechanism for the reaction between the following ester and methanol in the presence of NaOH.arrow_forwardSketch the mechanism (nucleophilic aromatic substitution) for the conversion of 2-iodobenzoic acid to N-phenylanthranilic acidarrow_forwardTranslated version of the problem: Write the obtainment of cinnamic acid (3-phenylpropenoic acid) using benzaldehyde and other necessary reagents, showing the reaction mechanism. can you solve question 5 ?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:9781305577190
Author:Kenneth L. Williamson, Katherine M. Masters
Publisher:Brooks Cole
Seven Name Reactions in One - Palladium Catalysed Reaction (047 - 053); Author: Rasayan Academy - Jagriti Sharma;https://www.youtube.com/watch?v=5HEKTpDFkqI;License: Standard YouTube License, CC-BY