
(a)
Interpretation:
To predict the products of the following transesterification reaction.
Concept Introduction:
Transesterification is the process of formation of a new ester molecule from the reaction of alcohol and an ester. This is like hydrolysis of ester but here nucleophile is alcohol molecule instead of
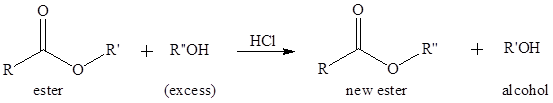
(b)
Interpretation:
To predict the products of the following transesterification reaction.
Concept Introduction:
Transesterification is the process of formation of a new ester molecule from the reaction of alcohol and an ester. This is like hydrolysis of ester but here nucleophile is alcohol molecule instead of
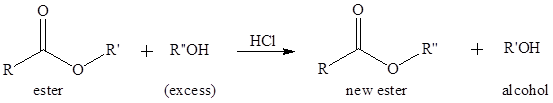
Want to see the full answer?
Check out a sample textbook solution
Chapter 11 Solutions
Essential Organic Chemistry (3rd Edition)
- One possible side-reaction of this experiment is biphenyl, explain how it can be formed. Here is the oringial reaction bromobenzene ----->magnesium. product is benze ring with MgBr attached to ringarrow_forwardWhat is the order of reactivity of SN1 and SN2 of n-butyl chloride, n-butyl bromide, sec-butyl chloride, tert-butyl chloride and crotyl chloride. Why? Sn2- with NaI/acetone and Sn1- with AgNO3/ethanolarrow_forwardIn this study, the researcher compared S N 2 and E2reaction rates for four substrates. Three of the substrates had a second halogen on the bposition in the molecule. This work also compared the behavior of two nucleophiles:dianion I and II. You should read the abstract and look at Scheme 1 (p. 3082) and Table 4(p. 3086). Abstract: The gas-phase reactions of benzoate and phenolate containing dianions with a series of ‚-substitutedalkyl bromides (X-CH2CH2Br, X ) H, F, Cl, Br) have been studied in a quadrupole ion trap mass spectrometer.Branching ratios between SN 2 and E2 products were measured and rate constants were determined. The‚-halogens increase both the S N 2 and E2 rates, but the effect is greater for the latter process and thereforethese substituents lead to an increase in the amount of elimination. The kinetic data for the SN 2 reactions canbe analyzed via a two-parameter, linear free-energy relationship and the results indicate that field-effects (i.e.,electron-withdrawing…arrow_forward
- In this study, the researcher compared S N 2 and E2reaction rates for four substrates. Three of the substrates had a second halogen on the bposition in the molecule. This work also compared the behavior of two nucleophiles:dianion I and II. You should read the abstract and look at Scheme 1 (p. 3082) and Table 4(p. 3086). Abstract: The gas-phase reactions of benzoate and phenolate containing dianions with a series of ‚-substitutedalkyl bromides (X-CH2CH2Br, X ) H, F, Cl, Br) have been studied in a quadrupole ion trap mass spectrometer.Branching ratios between SN 2 and E2 products were measured and rate constants were determined. The‚-halogens increase both the S N 2 and E2 rates, but the effect is greater for the latter process and thereforethese substituents lead to an increase in the amount of elimination. The kinetic data for the SN 2 reactions canbe analyzed via a two-parameter, linear free-energy relationship and the results indicate that field-effects (i.e.,electron-withdrawing…arrow_forwardIn this study, the researcher compared S N 2 and E2reaction rates for four substrates. Three of the substrates had a second halogen on the bposition in the molecule. This work also compared the behavior of two nucleophiles:dianion I and II. You should read the abstract and look at Scheme 1 (p. 3082) and Table 4(p. 3086). Abstract: The gas-phase reactions of benzoate and phenolate containing dianions with a series of ‚-substitutedalkyl bromides (X-CH2CH2Br, X ) H, F, Cl, Br) have been studied in a quadrupole ion trap mass spectrometer.Branching ratios between SN 2 and E2 products were measured and rate constants were determined. The‚-halogens increase both the S N 2 and E2 rates, but the effect is greater for the latter process and thereforethese substituents lead to an increase in the amount of elimination. The kinetic data for the SN 2 reactions canbe analyzed via a two-parameter, linear free-energy relationship and the results indicate that field-effects (i.e.,electron-withdrawing…arrow_forwardWhy is diethyl ether used as a solvent for the reduction reaction of the keystone (3,3-dimethyl-2-butanone) utilizing the reducing agent sodium borohydride (NaBH4). what is the purpose of was repeated washes with water? What is the purpose of repeated washes with brine?arrow_forward
- A chemist wanted to synthesize the anesthetic 2-ethoxy-2-methylpropane. He used ethoxide ion and 2-chloro-2-methylpropane for his synthesis and ended up with no ether. What was the product of his synthesis? What reagents should he have used?arrow_forwardAcid Halide Preparation reaction mechanism: Please use HCl and SOCl2 as reagentsarrow_forwardA greener alternative to bromination with elemental bromine is the reaction of the acetanilide with potassium bromide and ammonium ceric nitrate. How does this reaction work?arrow_forward
- Why is potassium hydroxide added to the reaction mixture? Willaimson Ether Synthesis a) To deprotonate the methylene group of the chloroacetic acid b) To deprotonate the methyl group of the cresol c) To generate the phenolate ionarrow_forward(a) Illustrate the following name reactions :(i) Hell-Volhard-Zelinsky reaction (ii) Wolff-Kishner reduction reaction(b) How are the following conversions carried out:(i) Ethylcyanide to ethanoic acid (ii) Butan-l-ol to butanoic acid(iii) Methylbenzene to benzoic acidWrite chemical equations for the involved reactions.arrow_forwardIn a Grignard synthesis of benzoic acid experiment, a student determines that their crude product was a mixture of benzoic acid and benzene. Describe a series of extractions using the following solvents and solutions to get pure benzoic acid or its salt in an ether solution. A. Methyl tert-butyl ether (MTBE) B. 3M HCl C. 3M NaOHarrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
