
(a)
Interpretation:
For the given alloy, an artificial age-hardening heat treatment needs to be recommended.
Concept Introduction:
The mixture of magnesium with other elements is known as magnesium alloy. The other elements include Zinc, Manganese, Aluminum and Copper.
Solidification takes place after complete mixing. It gets phase transformation to Liquid phase and there is no diffusion in the solid phase. With a decrease in the temperature of aluminum, the solubility of aluminium in magnesium also decreases.
If temperature is increased above,
Answer to Problem 12.30P
For artificial aging, the temperatures of solvus and eutectic point is determined. For this the curve between the percentage of weight of magnesium and the temperature needs to be drawn.
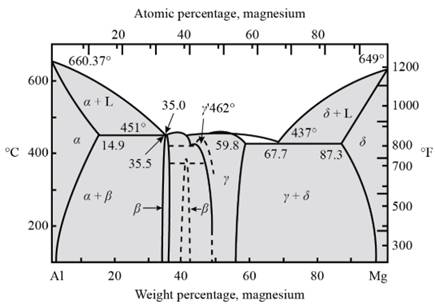
Fig. 1- Phase diagram of Al-Mg system
Explanation of Solution
Form the above graph, one gets to know all the temperatures for artificial age hardening of Magnesium-Aluminium alloy
| Temperature | Al- Mg | Al- Mg | Al- Mg |
| Treat at | Quenching | Quenching | Quenching |
| Age at | Temperature below | Temperature below | Temperature below |
Table No.
Thus, artificial age hardening depends on temperature of solid solubility and the eutectic point. Precipitation treat hardening is done by the help of quenching process.
(b)
Interpretation:
The amount of beta precipitates in all weight percentage of magnesium needs to be compared.
Concept Introduction:
The mixture of magnesium with other elements is known as magnesium alloy. The other elements include Zinc, Manganese, aluminum and Copper.
Solidification takes place after complete mixing. It gets phase transformation to Liquid phase and there is no diffusion in the solid phase. With a decrease in the temperature of aluminum, the solubility of aluminum in magnesium also decreases.
If temperature is increased above,
Answer to Problem 12.30P
The amount of beta precipitates in Al-
Explanation of Solution
Consider,
Aging temperature -
Tie line is passing through weight percentage of Mg as -
Hence, now one can calculate beta precipitate as −
For Al-
For Al-
And for Al-
From calculations, one can observe that if the weight percentage of magnesium in aluminum alloy increases then the amount of beta precipitate also increases.
(c)
Interpretation:
The requirement which is not satisfied in age hardening needs to be explained.
Concept Introduction:
The mixture of magnesium with other elements is known as magnesium alloy. The other elements include Zinc, Manganese, Aluminum and Copper.
Solidification takes place after complete mixing. It gets phase transformation to Liquid phase and there is no diffusion in the solid phase. With a decrease in the temperature of aluminum, the solubility of aluminum in magnesium also decreases.
If temperature is increased above,
Answer to Problem 12.30P
After the heat treatment, it can be observed that there is a little strengthening remains because the coherent precipitation is not formed.
Explanation of Solution
Age hardening is used to increase the yield strength of malleable metals like as −
- Aluminium
- Magnesium
- Nickel
- Titanium
The hardening depends on the temperature and its solubility in solid phase which results into pure or fine particles with the removal of the impurity phase. For precipitation, sometimes the alloy is kept at the elevated temperature for hours. This process of time delay is known as Aging.
After testing of the alloys with heat treatment, some amount of strengthening is observed. At this stage there is no formation of coherent precipitation. There is only simple dispersion strength as compared to the age hardening.
Want to see more full solutions like this?
Chapter 12 Solutions
Essentials Of Materials Science And Engineering
 MATLAB: An Introduction with ApplicationsEngineeringISBN:9781119256830Author:Amos GilatPublisher:John Wiley & Sons Inc
MATLAB: An Introduction with ApplicationsEngineeringISBN:9781119256830Author:Amos GilatPublisher:John Wiley & Sons Inc Essentials Of Materials Science And EngineeringEngineeringISBN:9781337385497Author:WRIGHT, Wendelin J.Publisher:Cengage,
Essentials Of Materials Science And EngineeringEngineeringISBN:9781337385497Author:WRIGHT, Wendelin J.Publisher:Cengage, Industrial Motor ControlEngineeringISBN:9781133691808Author:Stephen HermanPublisher:Cengage Learning
Industrial Motor ControlEngineeringISBN:9781133691808Author:Stephen HermanPublisher:Cengage Learning Basics Of Engineering EconomyEngineeringISBN:9780073376356Author:Leland Blank, Anthony TarquinPublisher:MCGRAW-HILL HIGHER EDUCATION
Basics Of Engineering EconomyEngineeringISBN:9780073376356Author:Leland Blank, Anthony TarquinPublisher:MCGRAW-HILL HIGHER EDUCATION Structural Steel Design (6th Edition)EngineeringISBN:9780134589657Author:Jack C. McCormac, Stephen F. CsernakPublisher:PEARSON
Structural Steel Design (6th Edition)EngineeringISBN:9780134589657Author:Jack C. McCormac, Stephen F. CsernakPublisher:PEARSON Fundamentals of Materials Science and Engineering...EngineeringISBN:9781119175483Author:William D. Callister Jr., David G. RethwischPublisher:WILEY
Fundamentals of Materials Science and Engineering...EngineeringISBN:9781119175483Author:William D. Callister Jr., David G. RethwischPublisher:WILEY





