
(a)
Interpretation:
The temperature at which austenite begins to transform needs to be determined.
Concept Introduction:
Austenite is defined as a gamma-phase iron, it is a metallic, non-magnetic iron allotrope or a solid solution of iron, containing an alloying element. Austenite which is known to exist above the eutectoid temperature of 1000K of plain carbon steel. Other alloys of the steel contain different eutectoid temperatures. Austenite can remain stable at room temperature only in the presence of austenite stability elements, e.g. Ni in adequate quantity.
Answer to Problem 12.63P
The temperatutre at which austenite will transform in Fe-
Explanation of Solution
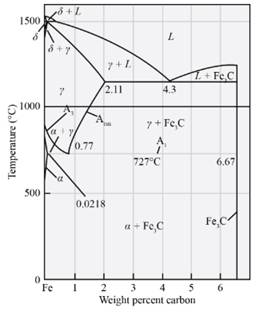
From the diagram cited above, the temperature at which austenite will start transforming on cooling in the Fe
(b)
Interpretation:
The primary microconstituents that are formed needs to be determined.
Concept Introduction:
The microconstituent of iron carbide includes austenite (
Answer to Problem 12.63P
The primary micro constituents that is formed in the alloy is primary Fe3C.
Explanation of Solution
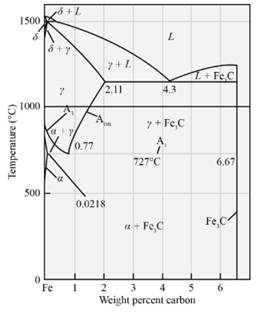
From the diagram mentioned above the microconstituent which is formed in the Fe-
(c)
Interpretation:
The amount and composition of phase at
Concept Introduction:
The plain iron-carbon alloys contain the amount of steel between 0.002% and 2.14 % by weight. At atmospheric pressure, the phases of iron plays a vital role because of the differences in carbon which forms different types of steel. High pressure phases of iron are significant as the prototype of the solid parts regarding planetary cores. The standard pressure phases are as follows:
1) Delta iron
2) Gamma iron/ austenite
3) Beta iron
4) Alpha iron
Answer to Problem 12.63P
The amount and composition of phases in Fe-
Explanation of Solution
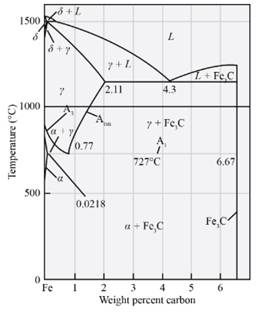
Draw a line at which passes through Fe3Cand ofFe3C and r.
(d)
Interpretation:
The amount and composition of phases at
Concept Introduction:
The plain iron-carbon alloys contain the amount of steel between 0.002% and 2.14 % by weight. At atmospheric pressure, the phases of iron plays a vital role because of the differences in carbon which forms different types of steel. High pressure phases of iron are significant as the prototype of the solid parts regarding planetary cores. The standard pressure phases are as follows:
1) Delta iron
2) Gamma iron/ austenite
3) Beta iron
4) Alpha iron
Answer to Problem 12.63P
The amount and composition of phases at
Explanation of Solution
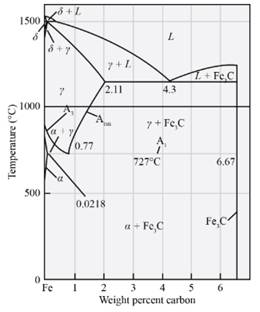
Based on the digram we have the formula as mentioned above,
(e)
Interpretation:
The amount and composition of microconstituent at
Concept Introduction:
The plain iron-carbon alloys contain the amount of steel between 0.002% and 2.14 % by weight. At atmospheric pressure, the phases of iron plays a vital role because of the differences in carbon which forms different types of steel. High pressure phases of iron are significant as the prototype of the solid parts regarding planetary cores. The standard pressure phases are as follows:
1) Delta iron
2) Gamma iron/ austenite
3) Beta iron
4) Alpha iron
Answer to Problem 12.63P
The amount and composition of microconstituent are
Explanation of Solution
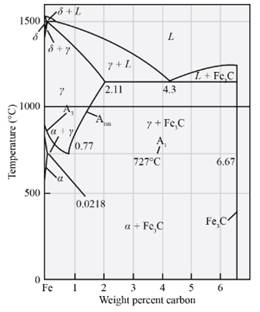
Based on the diagram mentioned above the microconstituent whichare present in the alloy at
The amount of primary Fe3C and composition are as follows:
Primary Fe3C=
% primary Fe3C=
The amount and composition of pearlite are:
Pearlite=
% pearlite=
Want to see more full solutions like this?
Chapter 12 Solutions
Essentials Of Materials Science And Engineering
 MATLAB: An Introduction with ApplicationsEngineeringISBN:9781119256830Author:Amos GilatPublisher:John Wiley & Sons Inc
MATLAB: An Introduction with ApplicationsEngineeringISBN:9781119256830Author:Amos GilatPublisher:John Wiley & Sons Inc Essentials Of Materials Science And EngineeringEngineeringISBN:9781337385497Author:WRIGHT, Wendelin J.Publisher:Cengage,
Essentials Of Materials Science And EngineeringEngineeringISBN:9781337385497Author:WRIGHT, Wendelin J.Publisher:Cengage, Industrial Motor ControlEngineeringISBN:9781133691808Author:Stephen HermanPublisher:Cengage Learning
Industrial Motor ControlEngineeringISBN:9781133691808Author:Stephen HermanPublisher:Cengage Learning Basics Of Engineering EconomyEngineeringISBN:9780073376356Author:Leland Blank, Anthony TarquinPublisher:MCGRAW-HILL HIGHER EDUCATION
Basics Of Engineering EconomyEngineeringISBN:9780073376356Author:Leland Blank, Anthony TarquinPublisher:MCGRAW-HILL HIGHER EDUCATION Structural Steel Design (6th Edition)EngineeringISBN:9780134589657Author:Jack C. McCormac, Stephen F. CsernakPublisher:PEARSON
Structural Steel Design (6th Edition)EngineeringISBN:9780134589657Author:Jack C. McCormac, Stephen F. CsernakPublisher:PEARSON Fundamentals of Materials Science and Engineering...EngineeringISBN:9781119175483Author:William D. Callister Jr., David G. RethwischPublisher:WILEY
Fundamentals of Materials Science and Engineering...EngineeringISBN:9781119175483Author:William D. Callister Jr., David G. RethwischPublisher:WILEY





