
Draw the organic products formed when cyclopentene is treated with each reagent. With some
reagents, no reaction occurs.
a.
b.
c.
d.
e.
f.
g.
h.
i.
j.
k.
l. Product in (k);
(a)
Interpretation: The organic product formed when cyclopentene is treated with
Concept introduction: The addition of
Answer to Problem 12.38P
The organic product formed when cyclopentene
Explanation of Solution
In the given reaction, when cyclopentene is treated with
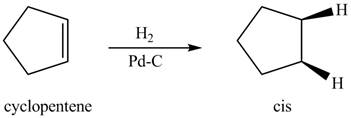
Figure 1
The organic product formed when cyclopentene
(b)
Interpretation: The organic product formed when cyclopentene is treated with Lindlar catalyst is to be predicted.
Concept introduction: The addition of
Answer to Problem 12.38P
Cyclopentene do not react with Lindlar catalyst.
Explanation of Solution
Alkenes do not react with Lindlar catalyst. Lindlar catalyst is used to reduce alkynes to
Cyclopentene do not react with Lindlar catalyst.
(c)
Interpretation: The product formed when the cyclopentene is treated with
Concept introduction: In presence of sodium metal in ammonia, the is alkyne is reduced to
Answer to Problem 12.38P
Cyclopentene do not react with
Explanation of Solution
Alkenes do not react with
Cyclopentene do not react with
(d)
Interpretation: The product formed when cyclopentene is treated with
Concept introduction: In presence of peroxide, alkene is oxidized to epoxide. This is known as epoxidation. This is a syn addition. The weak pi bond of alkene and weak
Answer to Problem 12.38P
The product formed when cyclopentene is treated with
Explanation of Solution
In the given reaction, when cyclopentene is treated with
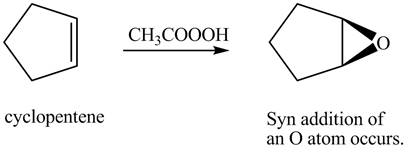
Figure 2
The product formed when cyclopentene is treated with
(e)
Interpretation: The product formed when cyclopentene is treated with
Concept introduction: Anti-dihroxylation takes place in two steps epoxidation followed by hydrolysis. In presence of peroxide, alkene is oxidized to epoxide. This is known as epoxidation. This is a syn addition. The weak pi bond of alkene and weak
Answer to Problem 12.38P
The product formed when cyclopentene is treated with
Explanation of Solution
In the given reaction, when cyclopentene is treated with
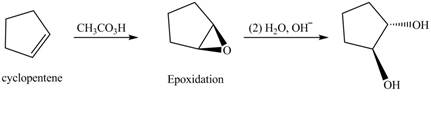
Figure 3
The product formed when cyclopentene is treated with
(f)
Interpretation: The product formed when cyclopentene is treated with
Concept introduction: Addition of two hydroxyl groups on double bond to form
Answer to Problem 12.38P
The product formed when cyclopentene is treated with
Explanation of Solution
In the given reaction, when cyclopentene is treated with
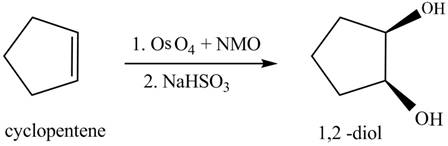
Figure 4
The product formed when cyclopentene is treated with
(g)
Interpretation: The product formed when cyclopentene is treated with
Concept introduction: Addition of two hydroxyl groups on double bond to form
Answer to Problem 12.38P
The product formed when cyclopentene is treated with
Explanation of Solution
In the given reaction, when cyclopentene is treated with
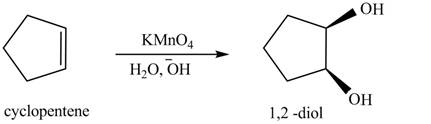
Figure 5
The product formed when cyclopentene is treated with
(h)
Interpretation: The product formed when cyclopentene is treated with
Concept introduction: The metal hydride reagents are good reducing agents such as
Answer to Problem 12.38P
Cyclopentene gives no reaction with
Explanation of Solution
The
Cyclopentene gives no reaction with
(i)
Interpretation: The product formed when cyclopentene is treated with
Concept introduction: In presence of ozone and
Answer to Problem 12.38P
The product formed when cyclopentene is treated with
Explanation of Solution
In the given reaction, when cyclopentene is treated with
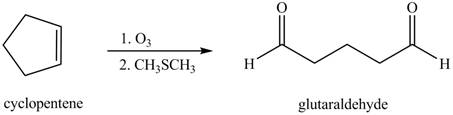
Figure 6
The product formed when cyclopentene is treated with
(j)
Interpretation: The product formed when cyclopentene is treated with
Concept introduction: Sharpless epoxidation involves the oxidation of double bond between carbon atoms to epoxide. This oxidation occurs only in allylic alcohol. This is an enantioselective oxidation, which means predominantly one enantiomer is formed. Sharpless reagents are
Answer to Problem 12.38P
The product formed when cyclopenteneis treated with
Explanation of Solution
There are two different chiral diethyl tartrate isomers,
When epoxidation is done using
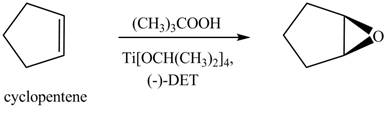
Figure 7
The product formed when cyclopenteneis treated with
(k)
Interpretation: The product formed when cyclopentene is treated with
Concept introduction: In presence of peroxide alkene is oxidized to epoxide this is known as epoxidation. This is a syn addition. The weak pi bond of alkene and weak
Answer to Problem 12.38P
The product formed when cyclopentene is treated with
Explanation of Solution
In the given reaction, when cyclopentene is treated with
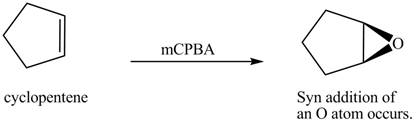
Figure 8
The product formed when cyclopentene is treated with
(l)
Interpretation: The product formed when cyclopentene is treated with
Concept introduction: In presence of peroxide, alkene is oxidized to epoxide this is known as epoxidation. This is a syn addition. The weak pi bond of alkene and weak
Answer to Problem 12.38P
The product formed when cyclopentene is treated with
Explanation of Solution
In the given reaction, when cyclopentene is treated with
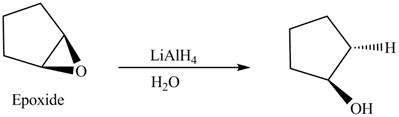
Figure 9
The product formed when cyclopentene is treated with
Want to see more full solutions like this?
Chapter 12 Solutions
ORG.CHEMISTRY CONNECT ACCESS>CUSTOM<
Additional Science Textbook Solutions
Fundamentals of Heat and Mass Transfer
Chemistry: Atoms First
Chemistry (7th Edition)
Thermodynamics, Statistical Thermodynamics, & Kinetics
CHEMISTRY-TEXT
The Organic Chem Lab Survival Manual: A Student's Guide to Techniques
- Need help with this Propose a preparation reaction for the formation of CH3NHCH2CH3 from organic compounds with only 1 or 2 carbonsarrow_forward4)Make the following starting from toluene or benzene, alcohols of 4 carbons or less, formaldehyde, ethylene oxide, or CO2:arrow_forwardThe compound para-dichlorobenzene is the active ingredient of the bathroom deodorizer "Albatross". How many distinct products are possible for the reaction of para-dichlorobenzene with 1 equivalent of HNO3 in H2SO4? a. 3 b. 2 c. 1 d. 4 e. 5arrow_forward
- In the reaction from compound 10 to compound 11, why the C=C bond is retained and is not hydrogenated? f) LiAlH4, Et2O, 08C; g) Ac2O, py, DMAP, CH2Cl2, RT, 77% over 2 steps;arrow_forward1. What is the alkane product from the reaction of C7H13COONa and NaOH? a. pentane b. hexane c. butane d. heptane 2. What is the resulting alkane if we have C5H11F and a C5H11F as reactants in Wurtz synthesis? a. hexane b. octane c. nonane d. decane 3.What is the resulting alkane if we have 2C2H5Cl as reactants in Wurtz Synthesis reaction? a. ethane b. butane c. hexane d. octanearrow_forwardCHEMISTRY AND ENVIRONMENTAL POLLUTION Explain how Hg2+ ion is transformed into methylmercury anddimethylmercury in the aquatic environment.arrow_forward
- The addition reaction of an acid (HBr) to an alkene (CH3CH=CH2) follows Markovnikov's rule and involves: A) initial attack by Br– B) initial attack by Br• C) isomerization of CH3CH2CH2Br D) formation of a primary carbocation. E) formation of a secondary carbonation. (F) Formation of allyl carbocationarrow_forwardMajor product and reactantsarrow_forwardHow does the first step in the reaction of propene with Br2 differ from the first step in the reaction of propene with HBr?arrow_forward
- 8. R-MgX and CO2 are reacted to form isobutyric acid. What is R? Use IUPAC nomenclature of the substituent. 9. From which cycloalkene can heptanoic acid be formed from through oxidative cleavage? Use IUPAC name. 10. The catalytic hydrogenation of a benzene will result to this cycloalkanearrow_forwardGive 5 differences of Terminal alkenes and internal alkenesarrow_forward1. What is the bond angle in carbon tetrachloride? 2. What is the product of the reaction of pent-2-ene with Cl2? 3.Which statement is correct? * A. The bond length increases when the difference between the electronegativity of the atoms is higher B. The bond length increases when the number when there is an increase in pi bonds. C. Increasing the bond polarity increases the bond length. D. Sp2 has shorter bond length that sp. 4. How many moles of O2 gas is theoretically needed for 1 mole of hexane? 5. What is the MOST LIKELY product of the reaction of pent-1-ene with HCL? 6. Which one creates a sigma bond? * A. 2 pz atomic orbitals B. 2 py atomic orbitals C. 2 px atomic orbitals D. none of these 7.How many hydrogen atoms are there in trans-1-bromo-2-methylcyclohexane? 8.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





