
Concept explainers
Draw the organic products formed when allylic alcoholA is treated with each reagent.
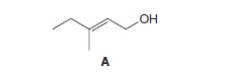
a.
b.
c.
d.
e.
f.
g. [1]
h.
(a)
Interpretation: The product formed when A is treated with
Concept introduction: The addition of
Answer to Problem 12.39P
The product formed when A is treated with
Explanation of Solution
When A is treated with
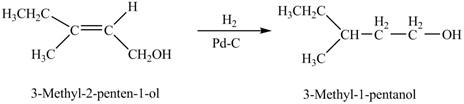
Figure 1
The product formed when A is treated with
(b)
Interpretation: The product formed when A is treated with
Concept introduction: In presence of peroxide alkene is oxidized to epoxide this is known as epoxidation. The weak pi bond of alkene and weak
Answer to Problem 12.39P
The product formed when A is treated with
Explanation of Solution
In the given reaction, when A is treated with
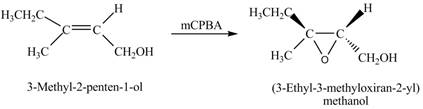
Figure 2
The product formed when A is treated with
(c)
Interpretation: The product formed when A is treated with
Concept introduction: Alcohols are oxidized to different carbonyl compounds depending upon the reagents and alcohol used. In presence of strong oxidizing reagents such as
Answer to Problem 12.39P
The product formed when A is treated with
Explanation of Solution
In the given reaction, when A is treated with
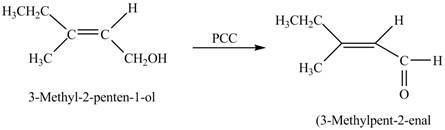
Figure 3
The product formed when A is treated with
(d)
Interpretation: The product formed when A is treated with
Concept introduction: Alcohols are oxidized to different carbonyl compounds depending upon the reagents and alcohol used. In the presence of strong oxidizing reagents such as
Answer to Problem 12.39P
The product formed when A is treated with
Explanation of Solution
In the given reaction, when A is treated with
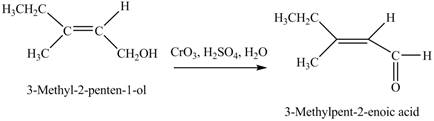
Figure 4
The product formed when A is treated with
(e)
Interpretation: The product formed when A is treated with Sharpless reagent
Concept introduction: Sharpless epoxidation involves the oxidation of double bond between carbon atoms to epoxide. This oxidation occurs only in allylic alcohol. This is an enantioselective oxidation, which means predominantly one enantiomer is formed. Sharpless reagents are
Answer to Problem 12.39P
The product formed when A is treated with Sharpless reagent
Explanation of Solution
There are two different chiral diethyl tartrate isomers,
When epoxidation is done using
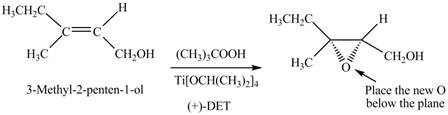
Figure 5
The product formed when A is treated with Sharpless reagent
(f)
Interpretation: The product formed when A is treated with Sharpless reagent
Concept introduction: Sharpless epoxidation involves the oxidation of double bond between carbon atoms to epoxide. This oxidation occurs only in allylic alcohol. This is an enantioselective oxidation, which means predominantly one enantiomer is formed. Sharpless reagents are
Answer to Problem 12.39P
The product of formed when A is treated with Sharpless reagent
Explanation of Solution
There are two different chiral diethyl tartrate isomers,
When epoxidation is done using
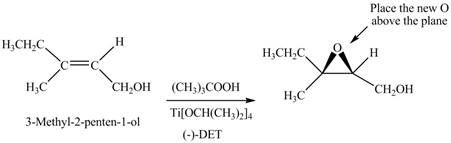
Figure 6
The product formed when A is treated with Sharpless reagent
(g)
Interpretation: The product formed when A is treated with given reagent is shown in Figure 7.
Concept introduction: Alcohols on treatment with phosphorus tribromide gives alkyl bromide. The
Answer to Problem 12.39P
The product formed in the given reaction is shown in Figure 7.
Explanation of Solution
The given reaction is,
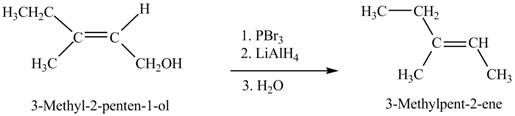
Figure 7
When the given alcohol is treated with
The product formed when A is treated with given reagent is shown in Figure 7.
(h)
Interpretation: The product formed when A is treated with
Concept introduction:
Answer to Problem 12.39P
The product formed when A is treated with
Explanation of Solution
In the given reaction, when A is treated with
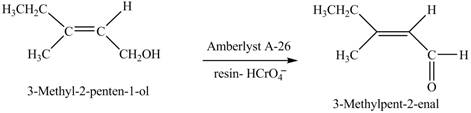
Figure 8
The product formed when A is treated with
Want to see more full solutions like this?
Chapter 12 Solutions
ORG.CHEMISTRY W/ACCESS+MODEL KIT PKG
Additional Science Textbook Solutions
Organic Chemistry As a Second Language: Second Semester Topics
Chemistry: Matter and Change
Chemistry
Chemistry & Chemical Reactivity
Chemistry (7th Edition)
Thermodynamics, Statistical Thermodynamics, & Kinetics
- Draw compounds that contain the following: (a) A primary alcohol (b) A tertiary nitrile (c) A secondary thiol (d) Both primary and secondary alcohols (e) An isopropyl group (f) A quaternary carbonarrow_forward1. What functional group is produced when an aldehyde reacts with H2/Pt? A.secondary alcohol B. carboxylic acid C.hemiacetal D. primary alcohol E.alkane F.tertiary alcohol G. alkene 2. What reaction occurs when an aldehyde reacts with H2/Pt to form a primary alcohol? A. Hydration B. Hydration C. Dehydration D. Oxidation E. Reduction( hydrogentation) 3. What reaction occurs when an Ester react with H+/H2O to from a carboxylic acid and alcohol? A. Dehydration B. Reduction ( Hydrogenation) C.Hydrolysis D. Hydration E.oxidationarrow_forwardDraw the products formed when phenol(C6H5OH) is treated with each reagent. Give an explanation. d. (CH3CH2)2CHCOCl, AlCl3 j. product in (d), then NH2NH2, – OHarrow_forward
- Molecule Type Boiling point (°C) CH3CH2CH3 Alkane -42 CH3CHO Aldehyde +21 CH3CH2OH Alcohol +78 i. Why is the boiling point of the aldehyde greater than that of the alkane?ii. Why is the boiling point of alcohol the highest?iii. Explain why the solubility of aldehydes and alcohols falls as the molecules get bigger.arrow_forwardDraw the products formed when phenol(C6H5OH) is treated with each reagent. Give an explanation. a. HNO3, H2SO4 h. product in (a), then Sn, HClarrow_forwarda. What is the chemical structure of biphenyl? b. Is it polar or nonpolar? _______________________ c. What is its water solubility in g/L? __________________________arrow_forward
- n-Butyl methyl ether is an isomer of MTBE and has a boiling point of 70 oC. Explain why the boiling point is significantly different compared to MTBE.arrow_forwardhow to convert 1-butanol into 4-heptanolarrow_forwardDraw the products formed when D-altrose is treated with each reagent. a. (CH3)2CHOH, HCl b. NaBH4, CH3OH c. Br2, H2O d. HNO3, H2O e. [1] NH2OH; [2] (CH3CO)2O, NaOCOCH3; [3] NaOCH3 f. [1] NaCN, HCl; [2] H2, Pd-BaSO4; [3] H3O+ g. CH3I, Ag2O h. C6H5CH2NH2, mild H+arrow_forward
- What is the correct reagent?arrow_forward-What is the IUPAC name of the product? a. Pentanoyl chloride b.Pentanoic acid chloride c. Pentanone chloride d. Pentanyl chloride -What type of reaction is illustrated in the reaction mechanism? a. Nucleophilic acyl substitution reaction b. Electrophilic substitution reaction c. Electrophilic acyl substitution reaction d. Nucleophilic addition reaction -What is the main functional group of the organic molecule? a. Carboxyl functional group b. Aldehyde functional group c. Carbonyl functional group d. Alcohol functional group -Aside from the organic molecule, what product is formed in the reaction? a. Hydronium ion b. Hydroxide anion c. Water molecule d. Hydroxyl group -The illustrated reaction mechanism is considered __________. a. Step-wise b. Unfavorable c. Concerted d. Continuous -Which atom in the reaction mechanism is the nucleophile? a. Chloride anion b. Carboxyl carbon c. Hydroxyl group d. Methyl group -What is the type of hybridization of the carboxyl carbon in the mechanism?…arrow_forwardExplain Addition of Alcohols—Acetal Formation ?arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


