
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
13th Edition
ISBN: 9780134421353
Author: Karen C. Timberlake
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 12, Problem 12.44APP
Classify each of the following alcohols as primary (1°), secondary (2°), or tertiary (3°): (12.2)
a. 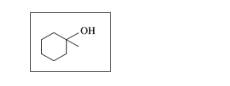
b.
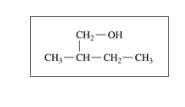
c.
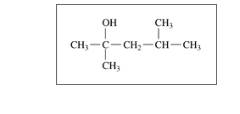
d.
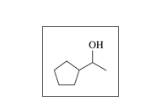
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
C6H8O6(aq) + C12H7O2NCL2(aq) ----> C6H6O6(aq) + C12H9O2NCL2(aq)
- C12H7O2NCL2 is abbreviated as DCIP
- Molar Lass of C6H8O6(s) is 176.14 g/mol
- Daily recommended intake (DRI) for adults: 90 mg of Vitamin C
Askor & Bich found that it required 23.14 mlof 1.32 x 10^-3 M DCIP soliution to reach the endpoint when titrating a 7.50ml aliquot of pear juice.
How many mg of ascorbic acid, C6H8O6 are present in the juice aliquot? _5.38_____(can you please show how to calculate this answer)
How many ml of this juice would Bich have to drink in order to meet the daily recommended intake (DRI)?__126__ (
Esterification Reaction:
12.00 grams of acetic acid is reacted with methanol and sulfuric acid catalyst under heated conditions. What amount (in grams) of the methanol is required for the reaction and what is the theoretical yield of the methyl acetate product? Include a balanced equation.
What is the cause of H2S as a gas while H2O is a liquid at room temperature of 25 ° C
Chapter 12 Solutions
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
Ch. 12.1 - Give the IUPAC name for each of the following: a....Ch. 12.1 - Give the IUPAC name for each of the following: a....Ch. 12.1 - Prob. 12.3PPCh. 12.1 - Draw the condensed structural formula, or...Ch. 12.1 - Give the common name for each of the following: a....Ch. 12.1 - Prob. 12.6PPCh. 12.1 - Draw the condensed structural formula, or...Ch. 12.1 - Draw the condensed structural formula, or...Ch. 12.2 - Classify each of the following alcohols as primary...Ch. 12.2 - Classify each of the following alcohols as primary...
Ch. 12.2 - Prob. 12.11PPCh. 12.2 - Prob. 12.12PPCh. 12.2 - Give an explanation for each of the following...Ch. 12.2 - Give an explanation for each of the following...Ch. 12.3 - Identify each of the following compounds as an...Ch. 12.3 - Identify each of the following compounds as an...Ch. 12.3 - Give the common name for each of the following: a....Ch. 12.3 - Give the common name for each of the following: a....Ch. 12.3 - Give the IUPAC name for each of the following: a....Ch. 12.3 - Give the IUPAC name for each of the following: a....Ch. 12.3 - Draw the condensed structural formula for each of...Ch. 12.3 - Draw the condensed structural formula for each of...Ch. 12.3 - Which compound in each of the following pairs...Ch. 12.3 - Which compound in each of the following pairs...Ch. 12.4 - Write the balanced chemical equation for the...Ch. 12.4 - Write the balanced chemical equation for the...Ch. 12.4 - Prob. 12.27PPCh. 12.4 - Draw the condensed structural or line-angle...Ch. 12.4 - Draw the condensed structural or line-angle...Ch. 12.4 - Draw the condensed structural or line-angle...Ch. 12.4 - Draw the condensed structural formulas for the...Ch. 12.4 - Draw the condensed structural formulas for the...Ch. 12.4 - Prob. 12.33PPCh. 12.4 - Prob. 12.34PPCh. 12.4 - Oxybenzone is an effective sunscreen whose...Ch. 12.4 - Avobenzone is a common ingredient in sunscreen....Ch. 12 - Prob. 12.37UTCCh. 12 - The compound frambinone has the taste of...Ch. 12 - A compound called resveratrol is an antioxidant,...Ch. 12 - A compound called cinnamaldehyde is found in...Ch. 12 - Prob. 12.41UTCCh. 12 - Prob. 12.42UTCCh. 12 - Prob. 12.43APPCh. 12 - Classify each of the following alcohols as primary...Ch. 12 - Give the IUPAC name for each of the following...Ch. 12 - Give the IUPAC name for each of the following...Ch. 12 - Draw the condensed structural or line-angle...Ch. 12 - Draw the condensed structural or line-angle...Ch. 12 - Which compound in each pair would be more soluble...Ch. 12 - Which compound in each pair would be more soluble...Ch. 12 - Draw the condensed structural or line-angle...Ch. 12 - Draw the condensed structural or line-angle...Ch. 12 - Draw the condensed structural or line-angle...Ch. 12 - Draw the condensed structural or line-angle...Ch. 12 - Give the IUPAC name for each of the following:...Ch. 12 - Give the IUPAC name for each of the following:...Ch. 12 - Draw the condensed structural or line-angle...Ch. 12 - Draw the condensed structural or line-angle...Ch. 12 - Which of the following aldehydes or ketones are...Ch. 12 - Which of the following aldehydes or ketones are...Ch. 12 - Draw the condensed structural or line-angle...Ch. 12 - Draw the condensed structural or line-angle...Ch. 12 - Prob. 12.63CPCh. 12 - Draw the condensed structural formulas and give...Ch. 12 - A compound with the formula C4H8O is synthesized...Ch. 12 - A compound with the formula C5H10O oxidizes to...Ch. 12 - Compound A is a primary alcohol whose formula is...Ch. 12 - Compound X is a secondary alcohol whose formula is...Ch. 12 - Prob. 21CICh. 12 - Prob. 22CICh. 12 - Prob. 23CICh. 12 - Prob. 24CICh. 12 - Prob. 25CICh. 12 - lonone is a compound that gives violets their...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- C7H6O3 + C4H6O3 <-> C9H8O4 + C2H4O2 1.0g of salicylic acid reacts completely with an recess if acetic anhydride. Whats the theoretical yield of aspirin in G?arrow_forwardA 70% aqueous solution of ethanol in water is commonly used in a laboratory as an antimicrobial solution. The antibacterial gel contains 80% ethanol. It is common to have access to 96% ethanol, answer the following: a.- Is 100% ethanol available? b.- How is it obtained? c.- Does the use of denatured absolute alcohol represent any danger?arrow_forwarda.Identify the functional groups in the ball-and-stick model of neral, a compound with a lemony odor isolated from lemongrass. b. Draw a skeletal structure of a constitutional isomer of neral that should be more water soluble. c.Label the most electrophillic carbon atom.arrow_forward
- 1.T/F California wines, unlike those in France, are not allowed to be blends of more than two varieties of grape. Select one: True Falsearrow_forwardWhich of the following statements is true16. lodoform, which has a molecular formula of CHal, is indicative that a ketomethyl group is present. 17. Based on the physical property, hydroquinone has a lower boiling point compared to catechol. 18. When FeCl3 is used in visualizing aspirin in thin layer chromatography, the resulting spot is red in color.arrow_forwardWhy CH3CH2NHCH3 has higher boiling point tha (CH3)3N even they have the same molecular weightarrow_forward
- Review the opening photograph about chocolate (which shows the structure of an active ingredient, theobromine) and then answer the following questions. (a) How do theobromine and caffeine differ structurally? (b) A 5.00-g sample of Hersheys cocoa contains 2.16% theobromine. What is the mass of the compound in the sample?arrow_forwardThe three-carbon diol used in antifreeze is It is nontoxic and is used as a moisturizing agent in foods. Oxidation of this substance within the liver produces pyruvic acid, which can be used by the body to supply energy. Give the structure of pyruvic acid.arrow_forwardBACTROBAN ointment contains 2% w/w mupirocin. H ow many grams of a polyethylene glycol ointment base must be mixed with the contents of a 22-g tube of the BACT ROBAN ointment to prepare one having a concentration of 5 mg/g?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry In FocusChemistryISBN:9781305084476Author:Tro, Nivaldo J., Neu, Don.Publisher:Cengage Learning
Chemistry In FocusChemistryISBN:9781305084476Author:Tro, Nivaldo J., Neu, Don.Publisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Chemistry In Focus
Chemistry
ISBN:9781305084476
Author:Tro, Nivaldo J., Neu, Don.
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning
07 Physical Properties of Organic Compounds; Author: Mindset;https://www.youtube.com/watch?v=UjlSgwq4w6U;License: Standard YouTube License, CC-BY