
Concept explainers
What
or
a. ![Chapter 12, Problem 12.46P, What alkene is needed to synthesize each 1,2-diol using the [1] OsO4 followed by NaHSO3 in H2O; or , example 1](http://dev-ingestion-image-output.s3-website-us-east-1.amazonaws.com/9780078021558/Chapter-12/images/21558-12-12.44p-question-digital_image001.jpg) b.
b. ![Chapter 12, Problem 12.46P, What alkene is needed to synthesize each 1,2-diol using the [1] OsO4 followed by NaHSO3 in H2O; or , example 2](http://dev-ingestion-image-output.s3-website-us-east-1.amazonaws.com/9780078021558/Chapter-12/images/21558-12-12.44p-question-digital_image002.jpg) c.
c. ![Chapter 12, Problem 12.46P, What alkene is needed to synthesize each 1,2-diol using the [1] OsO4 followed by NaHSO3 in H2O; or , example 3](http://dev-ingestion-image-output.s3-website-us-east-1.amazonaws.com/9780078021558/Chapter-12/images/21558-12-12.44p-question-digital_image003.jpg)
(a)
Interpretation: The alkene needed to synthesize the given
Concept introduction: Addition of two hydroxyl groups on double bond to form
In the presence of peroxide, alkene is oxidized to epoxide. This is known as epoxidation. This is a syn addition. The weak pi bond of alkene and weak
Answer to Problem 12.46P
The alkene needed to synthesize the given
Explanation of Solution
In presence of
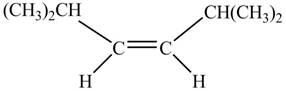
Figure 1
The corresponding chemical reaction is given below.
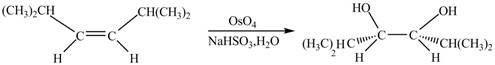
Figure 2
In presence of
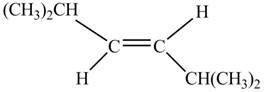
Figure 3
The corresponding chemical reaction is given below.
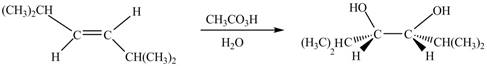
Figure 4
The alkene needed to synthesize the given
(b)
Interpretation: The alkene needed to synthesize the given
Concept introduction: Addition of two hydroxyl groups on double bond to form
In the presence of peroxide, alkene is oxidized to epoxide. This is known as epoxidation. This is a syn addition. The weak pi bond of alkene and weak
Answer to Problem 12.46P
The alkene needed to synthesize the given
Explanation of Solution
In presence of
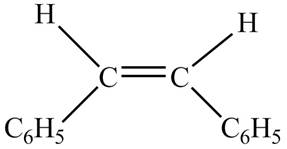
Figure 5
The chemical reaction is given below.
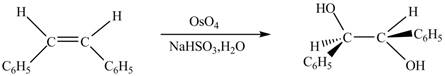
Figure 6
In presence of
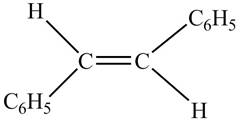
Figure 7
The chemical reaction is given below.
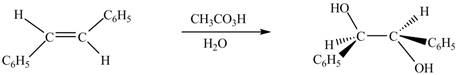
Figure 8
The alkene needed to synthesize the given
(c)
Interpretation: The alkene needed to synthesize the given
Concept introduction: Addition of two hydroxyl groups on double bond to form
In the presence of peroxide, alkene is oxidized to epoxide. This is known as epoxidation. This is a syn addition. The weak pi bond of alkene and weak
Answer to Problem 12.46P
The alkene needed to synthesize the given
Explanation of Solution
The given alkene is,
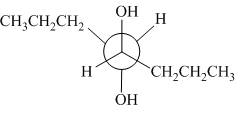
Figure 9
This is a staggered conformation in which two hydroxyl groups are anti to each other.
In presence of
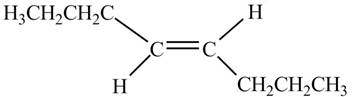
Figure 10
The chemical reaction is given below.
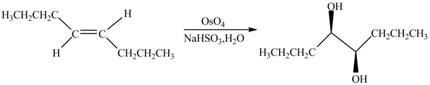
Figure 11
In presence of
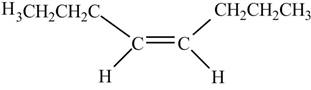
Figure 12
The chemical reaction is given below.
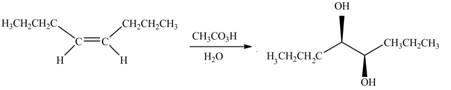
Figure 13
The alkene needed to synthesize the given
Want to see more full solutions like this?
Chapter 12 Solutions
Organic Chemistry
Additional Science Textbook Solutions
Chemistry (7th Edition)
Organic Chemistry - Standalone book
Introduction to Chemistry
Basic Chemistry (5th Edition)
Principles of General, Organic, Biological Chemistry
- A. OsO4 and NMO B. Br2 and H20 C. Hg(OAc)2, H2O and NaBH4, NaOH D. RCO3H E. BH3-THF and H2O2, NaOH Which reagent will complete this reaction?arrow_forward(a) Draw all constitutional isomers formed by monochlorination of each alkane with Cl2 and hν. (b) Draw the major monobromination product formed by heating each alkane with Br2.arrow_forwardOximene and myrcene, two hydrocarbons isolated from alfalfa that have the molecular formula C10H16, both yield 2,6- dimethyloctane when treated with H2 and a Pd catalyst. Ozonolysis of oximene forms (CH3)2C = O, CH2 = O, CH2(CHO)2, and CH3COCHO. Ozonolysis of myrcene yields (CH3)2C = O, CH2 = O, (two equiv), and HCOCH2CH2COCHO. Identify the structures of oximene and myrcene.arrow_forward
- (a) How will you convert:(i) Benzene to acetophenone (ii) Propanone to 2-Methylpropan-2-ol(b) Give reasons :(i) Electrophilic substitution in benzoic acid takes place at meta position.(ii) Carboxylic acids are higher boiling liquids than aldehydes, ketones and alcohols of comparable molecular masses.(iii) Propanal is more reactive than propanone in nucleophilic addition reactions.arrow_forwardDraw the products formed when phenol(C6H5OH) is treated with each reagent. Give an explanation. d. (CH3CH2)2CHCOCl, AlCl3 j. product in (d), then NH2NH2, – OHarrow_forwardDraw the structure of the major product of Benzene + HNO3 in H2SO4arrow_forward
- Give reasons for the following: (i) p-nitrophenol is more acidic than p-methylphenol. (ii) Bond length of C—O bond in phenol is shorter than that in methanol. (iii) (CH3)3C—Br on reaction with sodium methoxide (Na+ _OCH3) gives alkene as the main product and not an ether.arrow_forwardDraw the organic products formed when allylic alcohol A is treated with each reagent.a.H2 + Pd-C b.mCPBA c. PCC d.CrO3, H2SO4, H2O e.(CH3)3COOH, Ti[OCH(CH3)2]4, (+)-DET f. (CH3)3COOH, Ti[OCH(CH3)2]4, (−)-DET g. [1] PBr3; [2] LiAlH4; [3] H2O h.HCrO4−–Amberlyst A-26 resinarrow_forwardDraw the products formed when phenol(C6H5OH) is treated with each reagent. Give an explanation. a. HNO3, H2SO4 h. product in (a), then Sn, HClarrow_forward
- Draw the missing starting material. Reagent 1 is benzene and AlCl3. Reagent B is Zn(Hg) and HCl.arrow_forwardExplain why the addition of HBr to alkenes A and C is regioselective, forming addition products B and D, respectively.arrow_forwardExplain why the addition of HBr to alkenes A and C is regioselective,forming addition products B and D, respectively.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





