
Concept explainers
Explain why each name is incorrect and then write a correct name. Strategy: First draw the structural formula suggested by the incorrect name. Then identify the error, for example, failure to locate the longest chain that contains the
(a) 1-Methylpropene (b) 3-Pentene
(c) 2-Methylcyclohexene (d) 3,3-Dimethylpentene
(e) 4-Hexyne
(f) 2-lsopropyl-2-butene
(a)
Interpretation:
To analyse the error and write the correct IUPAC names.
Concept Introduction:
The rules to write the IUPAC names are as follows-
- The longest carbon chain is identified, and the root name is given accordingly.
- All the substituents attached to the root chain are determined.
- The position of the substituents is so assigned that the sum of their positions comes to be the least of all possible positions.
- The prefix di, tri, tetra is used if the same substituent is present two, three and four times respectively.
- All the substituents in the name are written in alphabetical order.
- For a cyclic hydrocarbon the prefix cyclo- is used.
Answer to Problem 16P
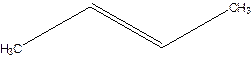
But-2-ene.
Explanation of Solution
1-Methylpropene.
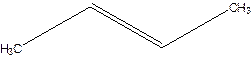
Error- longest chain not correctly located.
Correct name-
But-2-ene.
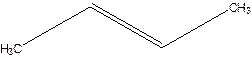
But-2-ene.
(b)
Interpretation:
To analyse the error and write the correct IUPAC names.
Concept Introduction:
The rules to write the IUPAC names are as follows-
- The longest carbon chain is identified, and the root name is given accordingly.
- All the substituents attached to the root chain are determined.
- The position of the substituents is so assigned that the sum of their positions comes to be the least of all possible positions.
- The prefix di, tri, tetra is used if the same substituent is present two, three and four times respectively.
- All the substituents in the name are written in alphabetical order.
- For a cyclic hydrocarbon the prefix cyclo- is used.
Answer to Problem 16P
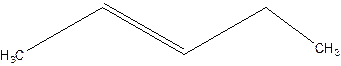
2-pentene.
Explanation of Solution
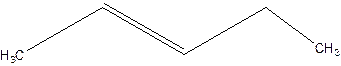
3-Pentene.
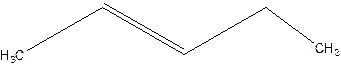
Error- Position of double bond not mentioned correctly.
Correct name-
2-Pentene.
(c)
Interpretation:
To analyse the error and write the correct IUPAC names.
Concept Introduction:
The rules to write the IUPAC names are as follows-
- The longest carbon chain is identified, and the root name is given accordingly.
- All the substituents attached to the root chain are determined.
- The position of the substituents is so assigned that the sum of their positions comes to be the least of all possible positions.
- The prefix di, tri, tetra is used if the same substituent is present two, three and four times respectively.
- All the substituents in the name are written in alphabetical order.
- For a cyclic hydrocarbon the prefix cyclo- is used.
Answer to Problem 16P
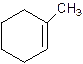
1-Methylcyclo-1-hexene.
Explanation of Solution
2-Methylcyclohexene.
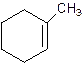
Error- Position of methyl not correct.
Correct name-
1-Methylcyclo-1-hexene.
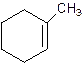
(d)
Interpretation:
To analyse the error and write the correct IUPAC names.
Concept Introduction:
The rules to write the IUPAC names are as follows-
- The longest carbon chain is identified, and the root name is given accordingly.
- All the substituents attached to the root chain are determined.
- The position of the substituents is so assigned that the sum of their positions comes to be the least of all possible positions.
- The prefix di, tri, tetra is used if the same substituent is present two, three and four times respectively.
- All the substituents in the name are written in alphabetical order.
- For a cyclic hydrocarbon the prefix cyclo- is used.
Answer to Problem 16P
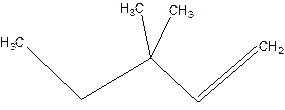
3,3-Dimethyl-1-pentene.
Explanation of Solution
3,3-Dimethylpentene.
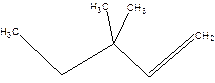
Error-position of double bond not mentioned.
Correct name-
3,3-Dimethyl-1-pentene.
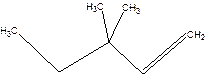
(e)
Interpretation:
To analyse the error and write the correct IUPAC names.
Concept Introduction:
The rules to write the IUPAC names are as follows-
- The longest carbon chain is identified, and the root name is given accordingly.
- All the substituents attached to the root chain are determined.
- The position of the substituents is so assigned that the sum of their positions comes to be the least of all possible positions.
- The prefix di, tri, tetra is used if the same substituent is present two, three and four times respectively.
- All the substituents in the name are written in alphabetical order.
- For a cyclic hydrocarbon the prefix cyclo- is used.
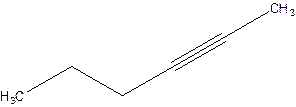
Answer to Problem 16P
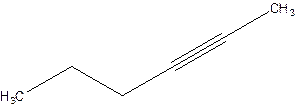
2-Hexyne.
Explanation of Solution
4-Hexyne.
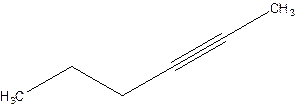
Error- position of triple bond is incorrectly mentioned.
Correct name 2-Hexyne.
(f)
Interpretation:
To analyse the error and write the correct IUPAC names.
Concept Introduction:
The rules to write the IUPAC names are as follows-
- The longest carbon chain is identified, and the root name is given accordingly.
- All the substituents attached to the root chain are determined.
- The position of the substituents is so assigned that the sum of their positions comes to be the least of all possible positions.
- The prefix di, tri, tetra is used if the same substituent is present two, three and four times respectively.
- All the substituents in the name are written in alphabetical order.
- For a cyclic hydrocarbon the prefix cyclo- is used.
Answer to Problem 16P
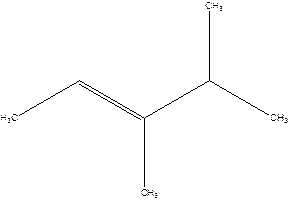
3,4-Dimethyl-2-pentene.
Explanation of Solution
2-Isopropyl-2-butene.
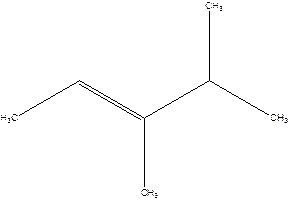
Error- longest chain is not correctly located.
The correct name will be-
3,4-Dimethyl-2-pentene.
Want to see more full solutions like this?
Chapter 12 Solutions
INTRO.TO GENERAL,ORGANIC+BIOCHEM.(LL)
- A structure with the formula C7H14O must be drawn that contains one stereocenter, an alcohol, and a disubstituted alkene including what the IUPAC name is for this compound. Dashes/wedges should be used to show which enantiomer is being drawn. In addition, what is the enantiomer drawing and IUPAC name of this compound, as well as the diastereomer of this compound and IUPAC name?arrow_forwardHello, just looking for help with my review. I'm supposed to draw a structural diagram for propyne, and methylpropyne. If im correct, would methylpropyne not just be 2-butyne? Thanksarrow_forwarddraw and name two structures that match the description of a trans-dihalocyclopentanearrow_forward
- The following name given is incorrect or incomplete, but it represents a real structure. Draw structure and name it correctly. (a) 5-chloro-4-methylhexanearrow_forwardWrite 2 equations for the dehydration of 2-methyl-2-butanol. One should show the structures of both alkenes, and the other one should be balanced.arrow_forwardSo, this is one of the questions on our quizzes. As far as I know, it's supposed to be 2-diethyl-5-methylhexane but it's not in the choices. Correct me if I am wrong. The quiz is about IUPAC naming of alkanes.arrow_forward
- The structure below has the formula C5H12 . Please name it according to IUPAC rules.arrow_forwardI need to use the IUPAC system to name my compund. Why is it wrong to name my compound 3-isopropylhexane? Could you please explain it step by step so that I can apply the naming technique correctly in future?arrow_forwardQuestion: In an organic chemistry laboratory, a student is performing a reaction to synthesize a compound. The reaction involves the conversion of an alcohol into an alkene using a dehydration process. The student chooses to use concentrated sulfuric acid (H2SO4) as the catalyst for this reaction. However, during the reaction, the student notices that another product is formed along with the desired alkene. (a) What is the likely identity of the unexpected product formed during the reaction? Explain your answer. (b) Provide a plausible explanation for the formation of the unexpected product and suggest a modification to the reaction conditions that would favor the desired alkene as the major product.arrow_forward
- a. Provide the IUPAC name for the following compound. Label any primary, secondary, or quaternary carbons, and primary, secondary or tertiary hydrogens. b. Draw a bond-line structure for 3,3-diethyl-2,5-dimethylnonane. c. The name 2-isopropyl-4-methylheptane provides enough information to draw its structure. However, it is NOT the correct IUPAC name. Explain why it is incorrect.arrow_forward5. How many isomers are there Of C6H14? 6. Aside from a halogenated alkane, what other product results from the bromination of hexane? Write the formulaarrow_forwardBased on the structures of these three molecules (3,3-dimethylbutan-2-one, 4-methylpentan-2-one, hexan-2-one), which molecule do you think will have the highest boiling point? The lowest? Will they be different or the same? Explain your logic.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





