
(a)
Interpretation:
The synthesis of the given product transformation should be identified.
Concept introduction:
Reduction:
Potassium tert-butoxide is a strong base, which used for the abstraction of acidic hydrogen from the molecule.
Alcohols is protected by using variety of reaction for example, Alcohols can be protected by treating with tosyl chloride in presence of base. Similarly it can be protected by using
Ozonolysis:
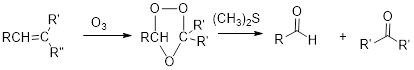
(b)
Interpretation:
The synthesis of the given product transformation should be identified.
Concept introduction:
Reduction: Aldehydes or ketones undergoing reduction by using reducing agent like
Potassium tert-butoxide is a strong base, which used for the abstraction of acidic hydrogen from the molecule.
The Grignard reaction:
Alkyl, vinyl, or aryl-magnesium halides (
Grignard reagent is reaction with carbonyl compound such as aldehyde or ketone, produces corresponding alcohol is the product and it is the one of the carbon – carbon bond forming reaction.
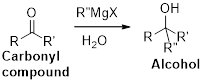
Tosylation reaction:
The alcohol is treated with any tosyl chloride (methane sulfonyl chloride) which yields tosylated product this reaction is called as alkyl tosylate and which is shown below,
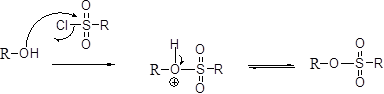
(c)
Interpretation:
The synthesis of the given product transformation should be identified.
Concept introduction:
Oxidation: Alcohols undergoing oxidation by using oxidizing agent like PCC (Pyridinium Chlorochromate) in dichloromethane which provides aldehyde.
Alcohols undergoing oxidation by using oxidizing agent like
SN2 reaction:
The alcohols is reaction with acids like hydrochloric acid or hydrobromic acid, the bromine atom attacks back side of the carbon atoms which is bearing alcohol group which yield the corresponding inversion product.
Tertiary alcohols undergo substitution very fast than the secondary alcohols because tertiary carbocation is more stable than the secondary carbocation than the primary carbocation.
(d)
Interpretation:
The synthesis of the given product transformation should be identified.
Concept introduction:
Oxidation: Alcohols undergoing oxidation by using oxidizing agent like PCC (Pyridinium Chlorochromate) in dichloromethane which provides aldehyde.
Alcohols undergoing oxidation by using oxidizing agent like
Alcohols is protected by using variety of reaction for example, Alcohols can be protected by treating with tosyl chloride in presence of base. Similarly it can be protected by using
SN2 reaction:
The alcohols is reaction with acids like hydrochloric acid or hydrobromic acid, the bromine atom attacks back side of the carbon atoms which is bearing alcohol group which yield the corresponding inversion product.
Tertiary alcohols undergo substitution very fast than the secondary alcohols because tertiary carbocation is more stable than the secondary carbocation than the primary carbocation.
The Grignard reaction:
Alkyl, vinyl, or aryl-magnesium halides (
Grignard reagent is reaction with carbonyl compound such as aldehyde or ketone, produces corresponding alcohol is the product and it is the one of the carbon – carbon bond forming reaction.
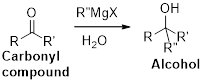
(e)
Interpretation:
The synthesis of the given product transformation should be identified.
Concept introduction:
Hydration:
When alkene is undergoes hydration with water in the presence of sulfuric acid which yields the alcohol. In this reaction, the water molecule will behave like a hydrogen halide to the alkene which gives the addition product this reaction is known as a hydration reaction.
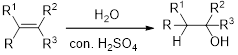
Alkene is reaction with water in the presence of sulfuric acid, first step is proton (
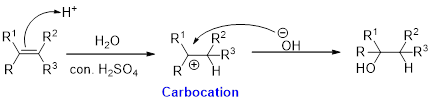
In hydration reaction, sulfuric acid is act as a proton donor, which is the driving force of the reaction. Hydration reaction will not go without acid (sulfuric acid).
Oxidation:
Alcohols undergoing oxidation by using oxidizing agent like
(f)
Interpretation:
The synthesis of the given product transformation should be identified.
Concept introduction:
Hydration:
When alkene is undergoes hydration with water in the presence of sulfuric acid which yields the alcohol. In this reaction, the water molecule will behave like a hydrogen halide to the alkene which gives the addition product this reaction is known as a hydration reaction.
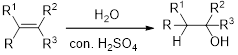
Alkene is reaction with water in the presence of sulfuric acid, first step is proton (

In hydration reaction, sulfuric acid is act as a proton donor, which is the driving force of the reaction. Hydration reaction will not go without acid (sulfuric acid).
Bromination:
Alcohols brominated by using
Alcohols undergoing oxidation by using oxidizing agent like
Potassium tert-butoxide is a strong base, which used for the abstraction of acidic hydrogen from the molecule.
The Grignard reaction:
Alkyl, vinyl, or aryl-magnesium halides (
Grignard reagent is reaction with carbonyl compound such as aldehyde or ketone, produces corresponding alcohol is the product and it is the one of the carbon – carbon bond forming reaction.
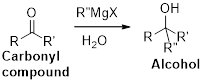
(g)
Interpretation:
The synthesis of the given product transformation should be identified.
Concept introduction:
Oxidation:
Alcohols undergoing oxidation by using oxidizing agent like
Hydration:
When alkene is undergoes hydration with water in the presence of sulfuric acid which yields the alcohol. In this reaction, the water molecule will behave like a hydrogen halide to the alkene which gives the addition product this reaction is known as a hydration reaction.
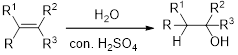
Alkene is reaction with water in the presence of sulfuric acid, first step is proton (

In hydration reaction, sulfuric acid is act as a proton donor, which is the driving force of the reaction. Hydration reaction will not go without acid (sulfuric acid).
The Grignard reaction:
Alkyl, vinyl, or aryl-magnesium halides (
Grignard reagent is reaction with carbonyl compound such as aldehyde or ketone, produces corresponding alcohol is the product and it is the one of the carbon – carbon bond forming reaction.
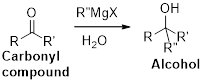
Dehydration reaction:
Removal of water molecule from the reaction when the alcohol is treated with strong acid like sulfuric acid.
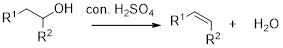
Alcohol is reaction with concentrated sulfuric acid, first alcohol gets protonated forms carbocation (more stable carbocation) followed by elimination of proton (
Tertiary carbocation is more stable than the secondary, secondary carbocation is more stable than primary.

In dehydration reaction, sulfuric acid is act as a proton donor, and which is used to protonate the alcohol and makes carbocation therefore sulfuric acid is the driving force of the reaction. Dehydration reaction will not go without acid (sulfuric acid).
(h)
Interpretation:
The synthesis of the given product transformation should be identified.
Concept introduction:
Alcohols undergoing oxidation by using oxidizing agent like
Reduction: Aldehydes or ketones undergoing reduction by using reducing agent like
The Grignard reaction:
Alkyl, vinyl, or aryl-magnesium halides (
Grignard reagent is reaction with carbonyl compound such as aldehyde or ketone, produces corresponding alcohol is the product and it is the one of the carbon – carbon bond forming reaction.
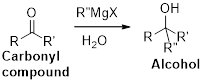
Kucherov Reaction:
Acetylene compounds Undergoes hydration with water which provides carbonyl compounds.
(i)
Interpretation:
The synthesis of the given product transformation should be identified.
Concept introduction:
Alcohols undergoing oxidation by using oxidizing agent like
Potassium tert-butoxide is a strong base, which used for the abstraction of acidic hydrogen from the molecule.
Hydration:
When alkene is undergoes hydration with water in the presence of sulfuric acid which yields the alcohol. In this reaction, the water molecule will behave like a hydrogen halide to the alkene which gives the addition product this reaction is known as a hydration reaction.
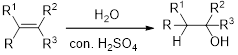
Alkene is reaction with water in the presence of sulfuric acid, first step is proton (

In hydration reaction, sulfuric acid is act as a proton donor, which is the driving force of the reaction. Hydration reaction will not go without acid (sulfuric acid).
The Grignard reaction:
Alkyl, vinyl, or aryl-magnesium halides (
Grignard reagent is reaction with carbonyl compound such as aldehyde or ketone, produces corresponding alcohol is the product and it is the one of the carbon – carbon bond forming reaction.
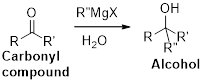
(j)
Interpretation:
The synthesis of the given product transformation should be identified.
Concept introduction:
The Grignard reaction:
Alkyl, vinyl, or aryl-magnesium halides (
Grignard reagent is reaction with carbonyl compound such as aldehyde or ketone, produces corresponding alcohol is the product and it is the one of the carbon – carbon bond forming reaction.
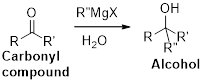
(k)
Interpretation:
The synthesis of the given product transformation should be identified.
Concept introduction:
Reduction: Aldehydes or ketones undergoing reduction by using reducing agent like
Dehydration reaction:
Removal of water molecule from the reaction when the alcohol is treated with strong acid like sulfuric acid.
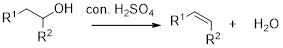
Alcohol is reaction with concentrated sulfuric acid, first alcohol gets protonated forms carbocation (more stable carbocation) followed by elimination of proton (
Tertiary carbocation is more stable than the secondary, secondary carbocation is more stable than primary.

In dehydration reaction, sulfuric acid is act as a proton donor, and which is used to protonate the alcohol and makes carbocation therefore sulfuric acid is the driving force of the reaction. Dehydration reaction will not go without acid (sulfuric acid).
(l)
Interpretation:
The synthesis of the given product transformation should be identified.
Concept introduction:
Reduction: Aldehydes or ketones undergoing reduction by using reducing agent like
Potassium tert-butoxide is a strong base, which used for the abstraction of acidic hydrogen from the molecule.
Tosylation reaction:
The alcohol is treated with any tosyl chloride (methane sulfonyl chloride) which yields tosylated product this reaction is called as alkyl tosylate and which is shown below,
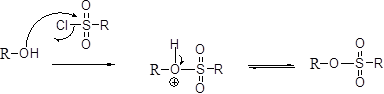
(m)
Interpretation:
The synthesis of the given product transformation should be identified.
Concept introduction
Reduction: Aldehydes or ketones undergoing reduction by using reducing agent like
Potassium tert-butoxide is a strong base, which used for the abstraction of acidic hydrogen from the molecule.
Tosylation reaction:
The alcohol is treated with any tosyl chloride (methane sulfonyl chloride) which yields tosylated product this reaction is called as alkyl tosylate and which is shown below,
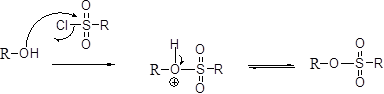
Hydroboration:
Hydroboration is the addition of a hydrogen-boron bond to the Carbon-Carbon, Carbon-Nitrogen, and Carbon-Oxygen double bonds and Carbon-Carbon triple bonds.
When alkene undergoes hydroboration using alkyl borane and hydrogen peroxide followed by hydrolysis which yields the alcohol. The formation of alcohol is depends on the less hindered carbon of the double bond.

(n)
Interpretation:
The synthesis of the given product transformation should be identified.
Concept introduction:
The Grignard reaction:
Alkyl, vinyl, or aryl-magnesium halides (
Grignard reagent is reaction with carbonyl compound such as aldehyde or ketone, produces corresponding alcohol is the product and it is the one of the carbon – carbon bond forming reaction.
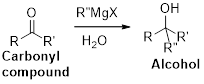
Dehydration reaction:
Removal of water molecule from the reaction when the alcohol is treated with strong acid like sulfuric acid.
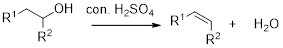
Alcohol is reaction with concentrated sulfuric acid, first alcohol gets protonated forms carbocation (more stable carbocation) followed by elimination of proton (
Tertiary carbocation is more stable than the secondary, secondary carbocation is more stable than primary.

In dehydration reaction, sulfuric acid is act as a proton donor, and which is used to protonate the alcohol and makes carbocation therefore sulfuric acid is the driving force of the reaction. Dehydration reaction will not go without acid (sulfuric acid).
(o)
Interpretation:
The synthesis of the given product transformation should be identified.
Concept introduction:
The Grignard reaction:
Alkyl, vinyl, or aryl-magnesium halides (
Grignard reagent is reaction with carbonyl compound such as aldehyde or ketone, produces corresponding alcohol is the product and it is the one of the carbon – carbon bond forming reaction.
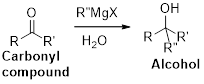
(p)
Interpretation:
The synthesis of the given product transformation should be identified.
Concept introduction:
Bromination:
Alcohols are reaction with PBr3 in pyridine which undergoes SN2 type of reaction, the bromine attacks the carbon atom through the back side and provides Inverse brominated compound.
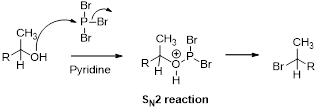
Reduction: Aldehydes or ketones undergoing reduction by using reducing agent like
(q)
Interpretation:
The synthesis of the given product transformation should be identified.
Concept introduction:
Hydroboration:
Hydroboration is the addition of a hydrogen-boron bond to the Carbon-Carbon, Carbon-Nitrogen, and Carbon-Oxygen double bonds and Carbon-Carbon triple bonds.
When alkene undergoes hydroboration using alkyl borane and hydrogen peroxide followed by hydrolysis which yields the alcohol. The formation of alcohol is depends on the less hindered carbon of the double bond.

Oxidation: Alcohols undergoing oxidation by using oxidizing agent like PCC (Pyridinium Chlorochromate) in dichloromethane which provides aldehyde.
Alcohols undergoing oxidation by using oxidizing agent like
The Grignard reaction:
Alkyl, vinyl, or aryl-magnesium halides (
Grignard reagent is reaction with carbonyl compound such as aldehyde or ketone, produces corresponding alcohol is the product and it is the one of the carbon – carbon bond forming reaction.
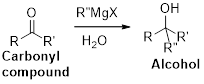
Want to see the full answer?
Check out a sample textbook solution
Chapter 12 Solutions
ORGANIC CHEMISTRY (LL) >CUSTOM PACKAGE<
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





