
Concept explainers
Interpretation:
A synthesis of hexyl butanoate using acetylene as only source of carbon atoms has to be proposed.
Concept introduction:
Markovnikov’s rule: unsymmetrical alkene reacts with hydrogen halide, halide ions goes to the more substitution position of carbon-carbon double bond which provides

Anti-Markovnikov’s rule: unsymmetrical alkene reacts with hydrogen halide, halide ions goes to the less substitution position of carbon-carbon double bond which provides alkyl halides.
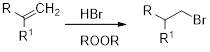
Hydroboration:
Hydroboration is the addition of a hydrogen-boron bond to the Carbon-Carbon, Carbon-Nitrogen, and Carbon-Oxygen double bonds and Carbon-Carbon triple bonds.
When alkene undergoes hydroboration using alkyl borane and hydrogen peroxide followed by hydrolysis which yields the alcohol. The formation of alcohol is depends on the less hindered carbon of the double bond.

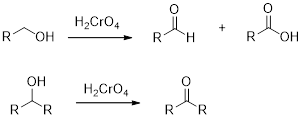
Chromic Acid:
Chromic Acid (
Lindlar reduction: The
Soda amide (
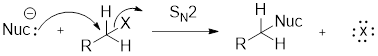
Structure of the substrate plays major role in the reactivity of
A nucleophile is an atom or molecule that donates an electron pair to make a covalent bond.
Want to see the full answer?
Check out a sample textbook solution
Chapter 12 Solutions
ORGANIC CHEMISTRY (LL) >CUSTOM PACKAGE<
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





