
Chemistry: An Introduction to General, Organic, and Biological Chemistry, Books a la Carte Edition (12th Edition)
12th Edition
ISBN: 9780321933331
Author: Karen C. Timberlake
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 12.3, Problem 12.21QAP
Which compound in each of the following pairs would be more soluble in water? Explain.
a. 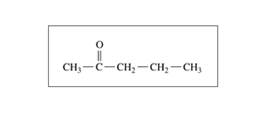
Or
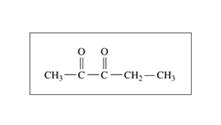
b. acetone or 2-pentanone
c. propanal or pentanal
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
(A)Complete reaction between benzil and sodium borohydride to from benzoin
b) complete reaction below between benzil and sodium borohydride to from hydrobenzoin
Write out the steps needed to separate hydrocarbon A and carboxylicacid B by using an extraction procedure.
Illustrate the Separation of cyclohexanamine and cyclohexanol by an extraction procedure ?
Chapter 12 Solutions
Chemistry: An Introduction to General, Organic, and Biological Chemistry, Books a la Carte Edition (12th Edition)
Ch. 12.1 - Give the IUPAC name for each of the following: a....Ch. 12.1 - Give the IUPAC name for each of the following: a....Ch. 12.1 - Prob. 12.3QAPCh. 12.1 - Draw the condensed structural formula, or...Ch. 12.1 - Prob. 12.5QAPCh. 12.1 - Prob. 12.6QAPCh. 12.1 - Draw the condensed structural formula, or...Ch. 12.1 - Draw the condensed structural formula, or...Ch. 12.2 - Prob. 12.9QAPCh. 12.2 - Prob. 12.10QAP
Ch. 12.2 - Prob. 12.11QAPCh. 12.2 - Prob. 12.12QAPCh. 12.2 - Prob. 12.13QAPCh. 12.2 - Give an explanation for each of the following...Ch. 12.3 - Give the common name for each of the following: a....Ch. 12.3 - Give the common name for each of the following: a....Ch. 12.3 - Prob. 12.17QAPCh. 12.3 - Prob. 12.18QAPCh. 12.3 - Draw the condensed structural formula for each of...Ch. 12.3 - Prob. 12.20QAPCh. 12.3 - Which compound in each of the following pairs...Ch. 12.3 - Which compound in each of the following pairs...Ch. 12.4 - Prob. 12.23QAPCh. 12.4 - Draw the condensed structural or line-angle...Ch. 12.4 - Prob. 12.25QAPCh. 12.4 - Prob. 12.26QAPCh. 12.4 - Draw the condensed structural formulas for the...Ch. 12.4 - Prob. 12.28QAPCh. 12.4 - Prob. 12.29QAPCh. 12.4 - Prob. 12.30QAPCh. 12.4 - Write the balanced chemical equation for the...Ch. 12.4 - Write the balanced chemical equation for the...Ch. 12 - Prob. 12.33UTCCh. 12 - The compound frambinone has the taste of...Ch. 12 - Prob. 12.35UTCCh. 12 - Prob. 12.36UTCCh. 12 - Prob. 12.37UTCCh. 12 - Prob. 12.38UTCCh. 12 - Prob. 12.39UTCCh. 12 - Prob. 12.40UTCCh. 12 - Prob. 12.41AQAPCh. 12 - Prob. 12.42AQAPCh. 12 - Prob. 12.43AQAPCh. 12 - Prob. 12.44AQAPCh. 12 - Draw the condensed structural or line-angle...Ch. 12 - Draw the condensed structural or line-angle...Ch. 12 - Prob. 12.47AQAPCh. 12 - Which compound in each pair would be more soluble...Ch. 12 - Prob. 12.49AQAPCh. 12 - Prob. 12.50AQAPCh. 12 - Prob. 12.51AQAPCh. 12 - Draw the condensed structural or line-angle...Ch. 12 - Prob. 12.53AQAPCh. 12 - Prob. 12.54AQAPCh. 12 - Draw the condensed structural or line-angle...Ch. 12 - Draw the condensed structural or line-angle...Ch. 12 - Which of the following aldehydes or ketones are...Ch. 12 - Which of the following aldehydes or ketones are...Ch. 12 - Prob. 12.59AQAPCh. 12 - Prob. 12.60AQAPCh. 12 - Prob. 12.61CQCh. 12 - Draw the condensed structural formulas and give...Ch. 12 - A compound with the formula C4H8O is synthesized...Ch. 12 - A compound with the formula C5H10O oxidizes to...Ch. 12 - Compound A is a primary alcohol whose formula is...Ch. 12 - Prob. 12.66CQCh. 12 - Prob. 21CICh. 12 - Prob. 22CICh. 12 - Prob. 23CICh. 12 - Prob. 24CICh. 12 - Prob. 25CICh. 12 - lonone is a compound that gives violets their...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Draw a stepwise mechanism for the sulfonation of an alkyl benzene such as A to form a substituted benzenesulfonic acid B. Treatment of B with base forms a sodium salt C that can be used as a synthetic detergent to clean away dirtarrow_forwardFormaldehyde levels above 0.10mg/1000L of ambient air can cause some humans to experience difficulty breathing. How many molecules of formaldehyde would be found in 1.0L of air at this concentration?arrow_forwardWhy are acetic acid, sodium acetate, and sodium caprate all soluble in water, whereas capric acid, a 10-carbon fatty acid, is not?arrow_forward
- Draw a stepwise mechanism for the sulfonation of an alkyl benzene such as A to form asubstituted benzenesulfonic acid B. Treatment of B with base forms a sodium salt C that canbe used as a synthetic detergent to clean away dirt.arrow_forwardExplain why methanol is insoluble in hexane than in ethanol or water using its modelarrow_forwardExplain how you would separate a mixture containing benzoic acid and a neutral substance, benzophenone. Both compounds are insoluble in water and soluble in ether. *Do not send flow charts. Please explainarrow_forward
- what would be the product? (see attached picture) A.) Salicytyl mathanoate B.) Methyl Salicylate C.) Methyl Benzoate D.) Phenyl Mathanoate E.) Methyl Phenolarrow_forwardWhich anion is more easily solvated by water? A OR Barrow_forwardExplain and show step by step how did the product formed when pentanol is treated with reagents in letter C and F only. this is about carboxylic acid and its derivative.arrow_forward
- We saw that it is necessary to use excess amine in the reaction of an acyl chloride with an amine. Explain why it is not necessary to use excess alcohol in the reaction of an acyl chloride with an alcohol.arrow_forwardBriefly explain why OtBu- sometimes favored over hydroxide as an elimination reagent?arrow_forwarda. Give at least three characteristics of dichloromethane that makes it a good extracting solvent for the alkaloid.b. Why is it necessary to remove a stopper from a separatory funnel when the liquid is being drained from it through a stopcock?c. What are emulsions? Why do they form during extractions? How is the formation of an emulsion minimized? How are emulsions removed?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning
Lipids - Fatty Acids, Triglycerides, Phospholipids, Terpenes, Waxes, Eicosanoids; Author: The Organic Chemistry Tutor;https://www.youtube.com/watch?v=7dmoH5dAvpY;License: Standard YouTube License, CC-BY