
Concept explainers
(a)
Interpretation:
The major product of the reaction of 2-methyl-2-butene with
Concept introduction:
Radical or free radical: unpaired valence electron of an atom, molecule, or ion is called as radical.
Bond strength is depends on the formation of the radical, if the radical is involving in resonance which is weakest bond strength.
Peroxide is a radical initiator, it has O-O bond in the molecule.
It is used in the radical reaction for the radical initiator.
Markovnikov’s rule:
(b)
Interpretation:
The major product of the reaction of 2-methyl-2-butene with
Concept introduction:
Radical or free radical: unpaired valence electron of an atom, molecule, or ion is called as radical.
Bond strength is depends on the formation of the radical, if the radical is involving in resonance which is weakest bond strength.
Peroxide is a radical initiator, it has O-O bond in the molecule.
It is used in the radical reaction for the radical initiator.
Markovnikov’s rule:
Alkene undergoes hydrohalogenation using hydrogen halide through breaking of carbon to carbon double bond, followed by the electrophilic addition of a hydrogen atom and halogen. The halide ion will add to the more substituted carbon in the alkene.
(c)
Interpretation:
The major product of the reaction of 2-methyl-2-butene with
Concept introduction:
Radical or free radical: unpaired valence electron of an atom, molecule, or ion is called as radical.
Bond strength is depends on the formation of the radical, if the radical is involving in resonance which is weakest bond strength.
Peroxide is a radical initiator, it has O-O bond in the molecule.
It is used in the radical reaction for the radical initiator.
Bromination of alkene in presence of peroxide:
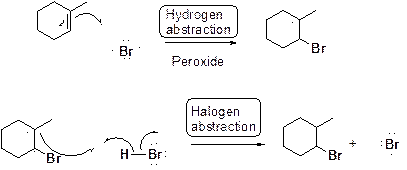
(d)
Interpretation:
The major product of the reaction of 2-methyl-2-butene with
Concept introduction:
Radical or free radical: unpaired valence electron of an atom, molecule, or ion is called as radical.
Bond strength is depends on the formation of the radical, if the radical is involving in resonance which is weakest bond strength.
Peroxide is a radical initiator, it has O-O bond in the molecule.
It is used in the radical reaction for the radical initiator.
Chlorination or iodination of alkene in presence of peroxide:
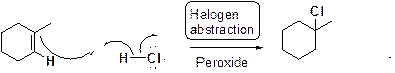
Want to see the full answer?
Check out a sample textbook solution
Chapter 12 Solutions
MODIFIED MASTERING CHEMISTRY W/ ETEXT
- Why is benzene a weak nucleophile?arrow_forwardIt is sometimes necessary to isomerize a cis alkene to a trans alkene in a synthesis, a process that cannot be accomplished in a single step. Using the reactions you have learned in Chapters 8–12, devise a stepwise method to convert cis-but-2-ene to trans-but-2-ene.arrow_forwardAccording to Zaitsev’s rule, which compound will be the major product for the elimination of 2-iodobutane? A. n-butane B. butene C. butan-2-ol D. but-2-enearrow_forward
- Draw the major products obtained from the reaction of one equivalent of HCl with the following compounds. For each reaction, indicate the kinetic andthermodynamic products. a. 2,3-dimethyl-1,3-pentadiene b. 2,4-dimethyl-1,3-pentadienearrow_forwardWhich reaction intermediate is formed when 4-methylcyclohexene reacts with Br2 dissolved in CCl4arrow_forwardPick the correct starting material and explain why the other ones are wrong and why the one picked is correct. Explain in detail with drawn out reactions.arrow_forward
- Industiral Chemistry Both Tetraethyl lead and methyl-t-butyl ether have been removed from or in the process of being removed from the gasoline additive industry. Explain why each of these compounds were used so highly, and why each is considered dangerous.arrow_forwardConsider the given alkyl halide with the chemical name, 2-Methyl-2-chlorobutane. What is the major product of the reaction if this alkyl halide was reacted with Ethanol (CH3CH2OH)? Product A Product B Product C Product Darrow_forward#20 B Draw structural formulas for all possible carbocations formed by the reaction of each alkene with HCl.arrow_forward
- What will be the color of the flame and the amount of soot if the following are ignited:(a) Hexane (b)Heptane(c) Cyclohexane (d) Cyclohexene (e) Benzene (f) Toluenearrow_forwardWhat is the major product obtained from hydroboration–oxidation of 2-methyl-2-butene? Draw the molecule.arrow_forwardwhat is the compound that most easily reacts with bromine in addition? a) CH4 b) C2H2 c) C3H8 d) C4H10arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


