
The graph shows how shows how
-
a. Why can Mg be used to reduce ZnO to Zn at all temperatures, but Zn cannot be used to reduce MgO to Mg at any temperature?
b. Why can C be used to reduce ZnO to Zn at some temperatures but not at others? At what temperatures can carbon be used to reduce zine oxide?
c. Is it possible to produce Zn from ZnO by its direct decomposition without requiring a coupled reaction? If so, at what approximate temperatures might this occur?
d. Is it possible to decompose CO to C end
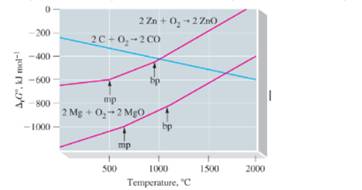
e. To the set of graphs, add straight lines representing the reactions
given that the three lines representing the formation of oxides of carbon intersect at about 800 eC. [Hint: At what other temperature can you relate and temperature?] The slopes of the three lines described above differ sharply Explain why this is so—that is, explain the slope of each line in terms of principles governing Gibbs energy change.
f. The graphs for the formation of oxides of other metals are similar to the ones shown for Zn and Mg: that is. they all have positive slopes Explain why carbon is such a good reducing agent for the reduction of metal oxides.
Want to see the full answer?
Check out a sample textbook solution
Chapter 13 Solutions
Mastering Chemistry With Pearson Etext -- Standalone Access Card -- For General Chemistry: Principles And Modern Applications (11th Edition)
- Many biochemical reactions that occur in cells require relatively high concentrations of potassium ion (K+). The concentration of K + in muscle cells is about 0.l5 M. The concentration of K+ in blood plasma is about 0.0050 M. The high internal concentration in cells is maintained by pumping K+ from the plasma. How much work must be done to transport 1.0 mole of K+ from the blood to the inside of a muscle cell at 37C, normal body temperature? When 1.0 mole of K+ is transferred from blood to the cells, do any other ions have to be transported? Why or why not?arrow_forwardChemists and engineers who design nuclear power plants have to worry about high-temperature reactions because it is possible for water to decompose. (a) Under what conditions does this reaction occur spontaneously? 2H2O(g) 2H2(g) + O2(g) (b) Under conditions where the decomposition of water is spontaneous, do nuclear engineers have to worry about an oxygen/hydrogen explosion? Justify your answer.arrow_forwardIodine, I2, dissolves readily in carbon tetrachloride. For this process, H = 0 kJ/mol. I2(s) I2 (in CCl4 solution) What is the sign of rG? Is the dissolving process entropy-driven or enthalpy-driven? Explain briefly.arrow_forward
- Estimate the temperature range over which each of the following reactions is spontaneous. (a) 2Al(s)+3Cl2(g)2AlCl3(s) (b) 2NOCl(g)2NO(g)+Cl2(g) (c) 4NO(g)+6H2O(g)4NH3(g)+5O2(g) (d) 2PH3(g)3H2(g)+2P(g)arrow_forwardLiquid water at 25C is introduced into an evacuated, insulated vessel. Identify the signs of the following thermodynamic functions for the process that occurs: H, S, Twater, Ssurr,, Suniv.arrow_forwardThe reaction shown below is involved in the refining of iron. (The table that follows provides all of the thermodynamic data you should need for this problem.) 2Fe2O3(s)+3C(s,graphite)4Fe(s)+3CO2(g) (a) Find H for the reaction. (b) S for the reaction above is 557.98 J/K. Find S° for Fe2O3(s). (c) Calculate G for the reaction at the standard temperature of 298 K. (There are two ways that you could do this.) (d) At what temperatures would this reaction be spontaneous? Compound Hf (kJ mol-1) S° (kJ mol-1) Gf (J mol-1 K-1) Fe2O3(s) -824.2 ? -742.2 C(s, graphite) 0 5.740 0 Fe(s) 0 27.3 0 CO2(g) -393.5 213.6 -394.4.83arrow_forward
- One of the important reactions in the biochemical pathway glycolysis is the reaction of glucose-6-phosphate (G6P) to form fructose-6-phosphate (F6P): G6PF6PG298=1.7kJ (a) Is the reaction spontaneous or nonspontaneous under standard thermodynamic conditions?. (b) Standard thermodynamic conditions imply the concentrations of G6P and F6P to be 1 M, however, in a typical cell, they are not even Close to these values. Calculate G when the concentrations of G6P and F6P are 120 M and 28 M respectively, and discuss the spontaneity of the forward reaction under these conditions. Assume the temperature is 37 C.arrow_forwardA reaction has H298=100 kj/mol and S298=250 J/mol K. Is the reaction spontaneous at room temperature? If not, under what temperature conditions will it become spontaneous?arrow_forwardConsider the reaction of 2 mol H2(g) at 25C and 1 atm with 1 mol O2(g) at the same temperature and pressure to produce liquid water at these conditions. If this reaction is run in a controlled way to generate work, what is the maximum useful work that can be obtained? How much entropy is produced in this case?arrow_forward
- The synthesis of glucose directly from CO2 and H2O and the synthesis of proteins directly from amino acids are both non-spontaneous processes under standard conditions. Yet it is necessary for these to occur for life to exist. In light of the second law of thermodynamics, how can life exist?arrow_forwardWhat is the third law of thermodynamics? What are standard entropy values, S, and how are these S values (listed in Appendix 4) used to calculate S for a reaction? How would you use Hesss law to calculate S for a reaction? What does the superscript indicate? Predicting the sign of S for a reaction is an important skill to master. For a gas-phase reaction, what do you concentrate on to predict the sign of S? For a phase change, what do you concentrate on to predict the sign of S? That is, how are Ssolid, Sliquid, and Sgas related to one another? When a solute dissolves in water, what is usually the sign of S for this process?arrow_forward
 Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning





