
(a)
Interpretation:
From mass balance 13.15, the equation 13-16 has to be derived.
Concept Introduction:
Ion-pairing in acid-base systems:
The possible ion-pair equilibria in the mixture of sodium hydrogen tartrate
(a)
Explanation of Solution
Derivation of equation 13-16 from mass balance 13-15:
Equation 13-15 (mass balance for sodium) is:
Equation 13-16 is:
Consider the two equations,
Substitute
Hence, the equation 13-16 is derived from mass balance 13.15.
(b)
Interpretation:
An expression for
Concept Introduction:
Mass balance equation for
The mass balance equation for
(b)
Answer to Problem 13.17P
The expression for
Explanation of Solution
Make the following substitutions to find expressions in terms of
Put these expressions into the mass balance equation:
Solve for
Hence, the expression for
The expression for
(c)
Interpretation:
Using the same approach in part (b), the expression for
Concept Introduction:
Mass balance equation for
The mass balance equation for
(c)
Answer to Problem 13.17P
The expression for
Explanation of Solution
To find
And now solve for
To find
Then keep these expressions into the mass balance:
And solve for
The expression for
(d)
Interpretation:
Using excel spreadsheet, the pH of the given solution has to be computed.
(d)
Answer to Problem 13.17P
The pH of the given solution is
Explanation of Solution
The spreadsheet used to compute pH of the given solution is shown in the below figure 1.
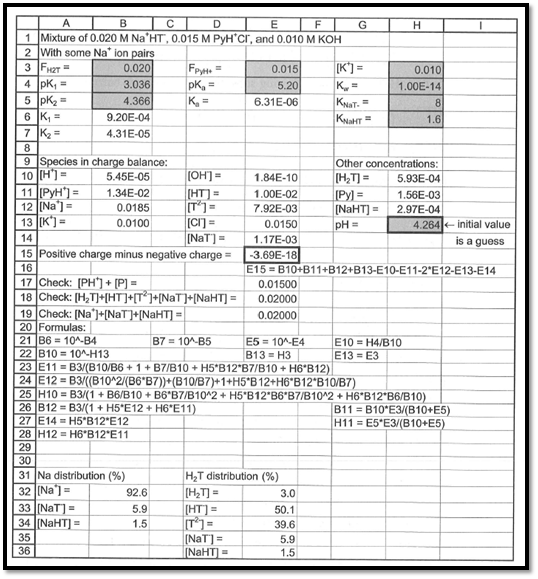
Figure 1
From the above spreadsheet, the pH of the solution is found as
The pH of the given solution is found out as
Want to see more full solutions like this?
Chapter 13 Solutions
QUANTIT.CHEM..(LL)-W/WEBASSIGN(6 MONTH)
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





