Expression for Kf' is as follows:
Kf'=(αY4−)(Kf) (1)
Here,
Kf' is conditional rate constant.
Kf is rate constant.
αY4− is fraction of free EDTA in form of Y4−.
Substitute 6.31×1018 for Kf and 3.7×10−7 for αY4− in equation (1).
Kf'=(6.31×1018)(3.7×10−7)=23347×108
At 0 mL of EDTA solution,
Expression to calculate pCu2+ is as follows:
pCu2+=−log[Cu2+] (2)
Substitute 0.0200 M for [Mn2+] in equation (2).
pCu2+=−log(0.0800 M)=1.09
When 0 mL of EDTA is added to Cu2+ solution, pCu2+ is 1.09.
At 20.0 mL of EDTA solution,
Expression to calculate total volume of solution is as follows:
Total volume of solution=(Volume of Cu2++Volume of EDTA) (3)
Substitute 25.0 mL for volume of Cu2+ and 20.0 mL for volume of EDTA in equation (3).
Total volume of solution=(25.0+20.0) mL=45 mL
Since EDTA forms strong metal complexes in the ratio of 1:1, moles of EDTA will be equivalent to moles of metal reacted.
Expression to calculate excess concentration of Cu2+ is as follows:
Excess [Cu2+]=(([Cu2+])(Volume of Cu2+)−([EDTA])(Volume of EDTA)Total volume of solution) (4)
Substitute 25.0 mL for volume of Cu2+, 0.0800 M for [Mn2+], 20.0 mL for volume of EDTA, 0.0400 M for [EDTA] and 45 mL for total volume of solution in equation (4).
Excess [Cu2+]=((0.0800 M)(25.0 mL)−(0.0400 M)(20.0 mL)45 mL)=0.02667 M
Substitute 0.02667 M for [Cu2+] in equation (2).
pCu2+=−log(0.02667 M)=1.57
At 40.0 mL of EDTA solution,
Substitute 25.0 mL for volume of Cu2+ and 40.0 mL for volume of EDTA in equation (3).
Total volume of solution=(25.0+40.0) mL=65 mL
Substitute 25.0 mL for volume of Cu2+, 0.0800 M for [Cu2+], 40.0 mL for volume of EDTA, 0.0400 M for [EDTA] and 65 mL for total volume of solution in equation (4).
Excess [Cu2+]=((0.0800 M)(25.0 mL)−(0.0400 M)(40.0 mL)65 mL)=0.00615 M
Substitute 0.00615 M for [Cu2+] in equation (2).
pCu2+=−log(0.00615 M)=2.21
At 49.0 mL of EDTA solution,
Substitute 25.0 mL for volume of Cu2+ and 49.0 mL for volume of EDTA in equation (3).
Total volume of solution=(25.0+49.0) mL=74 mL
Substitute 25.0 mL for volume of Cu2+, 0.0800 M for [Cu2+], 49.0 mL for volume of EDTA, 0.0400 M for [EDTA] and 74 mL for total volume of solution in equation (4).
Excess [Cu2+]=((0.0800 M)(25.0 mL)−(0.0400 M)(49.0 mL)74 mL)=0.000541 M
Substitute 0.000541 M for [Cu2+] in equation (2).
pCu2+=−log(0.000541 M)=3.27
At 50.0 mL of EDTA solution,
Formula to calculate molarity of solution is as follows:
Molarity of solution(M)=Moles of soluteVolume (L) of solution (5)
Rearrange equation (5) for moles of solute.
Moles of solute=[(Molarity of solution)(Volume of solution)] (6)
Substitute 0.0800 M for molarity and 25.0 mL for volume of solution in equation (6) to calculate millimoles of Cu2+.
Millimoles of Cu2+=(0.0800 M)(25.0 mL)=2 mmol
Substitute 0.0400 M for molarity and 50.0 mL for volume of solution in equation (6) to calculate millimoles of EDTA.
Millimoles of EDTA=(0.0400 M)(50.0 mL)=2 mmol
Substitute 25.0 mL for volume of Cu2+ and 50.0 mL for volume of EDTA in equation (3).
Total volume of solution=(25.0+50.0) mL=75 mL
Chemical reaction occurs as follows:
Cu2++Y2−↔CuY2−
Concentration of CuY2− at equivalence point is calculated as follows:
[CuY2−]=2 mmol75 mL=0.0267 M
Consider change in concentrations of ionic species to be negligible and amount of Cu2+ and EDTA to be x.
Expression to calculate Kf' at equivalence point is as follows:
Kf'=[CuY2−][Cu2+][EDTA] (7)
Substitute 0.0267 M for [CuY2−], x for [Cu2+], x for [EDTA] and 23347×108 for Kf' in equation (7).
23347×108=0.0267(x)(x)
Solve for x,
x=1.0694×10−7 M
Substitute 1.0694×10−7 M for [Cu2+] in equation (2).
pCu2+=−log(1.0694×10−7 M)=6.97
At 51.0 mL of EDTA solution,
Substitute 25.0 mL for volume of Cu2+ and 51.0 mL for volume of EDTA in equation (3).
Total volume of solution=(25.0+51.0) mL=76 mL
Chemical reaction occurs as follows:
Cu2++Y2−↔CuY2−
Concentration of CuY2− at equivalence point is calculated as follows:
[CuY2−]=2 mmol76 mL=0.0263 M
Concentration of excess EDTA is calculated as follows:
Excess [EDTA]=(0.0400 M)(1.0 mL)76 mL=0.000526 M
Rearrange equation (7) for [Cu2+].
[Cu2+]=[CuY2−]Kf'[EDTA] (8)
Substitute 0.0263 M for [CuY2−], 0.000526 M for [EDTA] and 23347×108 for Kf' in equation (8).
[Cu2+]=0.0263 M(23347×108)(0.000526 M)=2.142×10−11 M
Substitute 2.142×10−11 M for [Cu2+] in equation (2).
pCu2+=−log(2.142×10−11 M)=10.67
At 55.0 mL of EDTA solution,
Substitute 25.0 mL for volume of Cu2+ and 55.0 mL for volume of EDTA in equation (3).
Total volume of solution=(25.0+55.1) mL=80 mL
Chemical reaction occurs as follows:
Cu2++Y2−↔CuY2−
Concentration of CuY2− at equivalence point is calculated as follows:
[CuY2−]=2 mmol80 mL=0.025 M
Concentration of excess EDTA is calculated as follows:
Excess [EDTA]=(0.0400 M)(5.0 mL)80 mL=0.0025 M
Substitute 0.025 M for [CuY2−], 0.0025 M for [EDTA] and 23347×108 for Kf' in equation (8).
[Cu2+]=0.025 M(23347×108)(0.0025 M)=4.283×10−12 M
Substitute 4.283×10−12 M for [Cu2+] in equation (2).
pCu2+=−log(4.283×10−12 M)=11.37
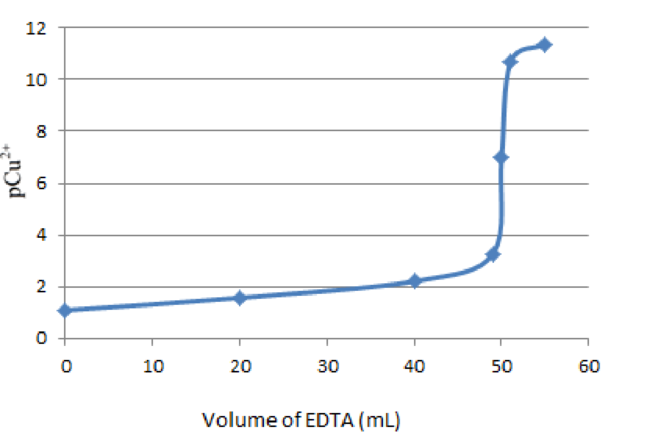
Graph of pCu2+ versus volume of EDTA or titrant added is as follows:


 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY




