
(a)
Interpretation:
The given reaction equation to be predicted
Concept Introduction:
Addition reaction: A general reaction type in which a substance X-Y adds to the multiple bond of an unsaturated reactant to yield a saturated product that has only single bonds.
Hydrogenation (Addition of
(b)
Interpretation:
The given reaction equation to be predicted
Concept Introduction:
Addition reaction: A general reaction type in which a substance X-Y adds to the multiple bond of an unsaturated reactant to yield a saturated product that has only single bonds.
Halogenation (Addition of halogen molecule to alkenes): The addition of halogen molecules like
c)
Interpretation:
The given reaction equation to be predicted
Concept Introduction:
Addition reaction: A general reaction type in which a substance X-Y adds to the multiple bond of an unsaturated reactant to yield a saturated product that has only single bonds.
Hydrohalogenation (Addition of hydrogen halide to alkenes): The addition of hydrogen halide to a multiple bond to give an
Markovnikov’s rule: In the addition of
(d)
Interpretation:
The given reaction equation to be predicted
Concept Introduction:
Addition reaction: A general reaction type in which a substance X-Y adds to the multiple bond of an unsaturated reactant to yield a saturated product that has only single bonds.
Hydration:
When alkene is undergoes hydration with water in the presence of sulfuric acid which yields the alcohol. In this reaction, the water molecule will behave like a hydrogen halide to the alkene which gives the addition product this reaction is known as a hydration reaction.
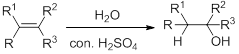
Alkene is reaction with water in the presence of sulfuric acid, first step is proton (
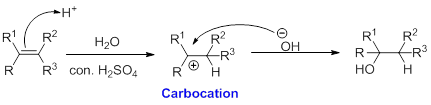
In hydration reaction, sulfuric acid is act as a proton donor, which is the driving force of the reaction. Hydration reaction will not go without acid (sulfuric acid).
Markovnikov’s rule: In the addition of
Want to see the full answer?
Check out a sample textbook solution
Chapter 13 Solutions
Modified Mastering Chemistry With Pearson Etext -- Standalone Access Card -- For Fundamentals Of General, Organic, And Biological Chemistry (8th Edition)
- Write the formulas of the four singly chlorinated isomers formed when 2-methylbutane reacts with Cl2 in the presence of light.arrow_forwarddraw the alkane strucutre based off the IR spectrum, 1H NMR spectrum, and 13C NMR spectrum for this compound.arrow_forwardDraw condensed structural formulas for the two carboxylic acids with the molecular formula C4H8O2arrow_forward
- In the preparation of aspirin, You can do the functional group test to ensure the completion of the reaction. (a) What is the name of reagent used? (b) What is your observation if any unreacted starting material is present? (c) What is the name of the functional group responsible for this reaction?arrow_forwardFor the following reaction, 4.91 grams of water are mixed with excess chlorine gas. The reaction yields 12.5 grams of hydrochloric acid.chlorine (g) + water (l) hydrochloric acid (aq) + chloric acid (HClO3) (aq) What is the theoretical yield of hydrochloric acid ? grams What is the percent yield of hydrochloric acid ? %arrow_forwardWhat is the empirical formula for C3H6O3? C3H6O3 C6H12O6 CH2O None of thesearrow_forward
- Compare the densities of low-density polyethylene (LDPE) and high-density poly- ethylene (HDPE) with the densities of the liquid alkanes.How might you account for the differences between them?arrow_forwardDimethyl ether has the same molecular formula as ethanol (Problem 4.57) but very different properties. Propose a structure for dimethyl ether in which the oxygen is bonded to two carbonsarrow_forwardEthylene glycol, the main ingredient in antifreeze, contains 38.7% carbon, 9.7% hydrogen and 51.6 % oxygen. Calculate the empirical and molecular formulas for ethylene glycol. Given the molar mass is approximately 60 g/mol. A) Empirical formula: B)Molecular formula: Explain how you obtained the Molecular formula (b)?arrow_forward
- The following equation shows the reaction of baking soda (NaHCO3) and hydrochloric acid (HCl). NaHCO3+HCl → CO2+H2O+NaCl If you have 3.0 grams of NaHCO3, how many moles of HCl are needed for a complete relation?arrow_forwardEthylene oxide is produced industrially from the reaction of ethylene with oxygen at atmospheric pressure and 277 oC, in the presence of silver catalyst.C2H4(g) + O2(g) → C2H4O(g) (unbalanced)Assuming 100 % yield, how many kg of ethylene oxide can be produced from 34600 L of a mixture containing ethylene and oxygen in 1:1 molar ratio?arrow_forwardGiven the balanced equation with an unknown compound represented by X, which compound is represented by X?arrow_forward
