
Concept explainers
(a)
Interpretation:
From the condensed structures for 2,3-dimethyl-2-pentene whether cis-trans isomers exist or not has to be given. And if it exists, both the isomers has to be drawn.
Concept Introduction:
Condensed structures: A condensed structures only shows the total number of atoms but not bonds exist between the atoms. The vertical and horizontal line which represents bonding all should be omitted.
Line structure: In organic compounds, the series of atoms in the compound are bonded together which shown by drawing a line between them. End of line segment represents carbon
Predicting cis-trans isomers:
In general, the
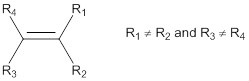
Cis-isomer: In cis-configuration, the two bulkier substituents of the double bond are on the same side.
Trans-isomer: In trans-configuration, the two bulkier substituents of the double bond are on the opposite side.
(b)
Interpretation:
From the both condensed and line structures for 2-Methyl-2-hexene whether cis-trans isomers exist or not has to be given. And if it exists, both the isomers has to be drawn.
Concept Introduction:
Condensed structures: A condensed structures only shows the total number of atoms but not bonds exist between the atoms. The vertical and horizontal line which represents bonding all should be omitted.
Line structure: In organic compounds, the series of atoms in the compound are bonded together which shown by drawing a line between them. End of line segment represents carbon
Cis-isomer: In cis-configuration, the two bulkier substituents of the double bond are on the same side.
Trans-isomer: In trans-configuration, the two bulkier substituents of the double bond are on the opposite side.
(c)
Interpretation:
From the line structures for 2-hexene whether cis-trans isomers exist or not has to be given. And if it exists, both the isomers has to be drawn.
Concept Introduction:
Condensed structures: A condensed structures only shows the total number of atoms but not bonds exist between the atoms. The vertical and horizontal line which represents bonding all should be omitted.
Line structure: In organic compounds, the series of atoms in the compound are bonded together which shown by drawing a line between them. End of line segment represents carbon
Cis-isomer: In cis-configuration, the two bulkier substituents of the double bond are on the same side.
Trans-isomer: In trans-configuration, the two bulkier substituents of the double bond are on the opposite side.
Want to see the full answer?
Check out a sample textbook solution
Chapter 13 Solutions
Modified Mastering Chemistry With Pearson Etext -- Standalone Access Card -- For Fundamentals Of General, Organic, And Biological Chemistry (8th Edition)
- How many of the carbons in the following structure are stereocenters?arrow_forwardWhat functional groups are present in the peaks?arrow_forwardThe explosive trinitrotoluene (TNT) is made by carrying out three successive nitration reactions on toluene. If these nitrations only occur in the ortho and para positions relative to the methyl group, what is the structure of TNT?arrow_forward
- The reaction of methoxy benzene with hydrogen iodide will yield a phenol and an alkyl halide. Which of following choices is the correct combination of the products?arrow_forwardWhich of the following Fischer formula is or are monosaccharide that has two chiral centers?arrow_forwardDraw condensed structural formulas for the two carboxylic acids with the molecular formula C4H8O2arrow_forward
- For A, B, C, D, E, F, identify the circled functional groups and linkages in the compound in the picture.arrow_forwardWhich of the following define the stereochemistry of alanine (as per the structure shown)? Note: Functional groups arranged horizontally are facing towards the front, and the functional groups arranged vertically are facing towards the back. a) d- b) S- c) R- d) I-arrow_forwardWhat is the “octet rule” and its biologically important exception?arrow_forward
- Glucose-1-phosphate has a ΔG°′ value of −20.9 kJ/mol, whereas that for glucose-6-phosphate is −12.5 kJ/mol. After reviewing the molecular structures of these compounds, explain why there is such a difference in these values.arrow_forwardWhat properties do each of the following R groups have?arrow_forwardWhat does it mean to describe a glycosidic bond as β-1,4-? To describe it as α-1,6-?arrow_forward
 Principles Of Radiographic Imaging: An Art And A ...Health & NutritionISBN:9781337711067Author:Richard R. Carlton, Arlene M. Adler, Vesna BalacPublisher:Cengage Learning
Principles Of Radiographic Imaging: An Art And A ...Health & NutritionISBN:9781337711067Author:Richard R. Carlton, Arlene M. Adler, Vesna BalacPublisher:Cengage Learning
