
Concept explainers
Interpretation: The fuel values of liquid propanol and isopropanol are to be calculated. The comparison of their fuel values is to be done.
Concept introduction: Fuel value is defined as the amount of energy which is generated by the complete combustion of a particular mass of the fuel. The fuel value of alcohols increases with increase in number of carbon atoms in the alcohol.
To determine: The fuel value of propanol and isopropanol and the comparison of their fuel values.
Answer to Problem 13.88QP
The fuel value of diethyl ether is
Explanation of Solution
The structure of propanol is,
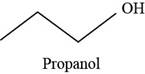
Figure 1
The structure of isopropanol is,
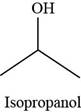
Figure 2
The fuel value is calculated by the formula,
Propanol (
According to the above reaction,
Molar mass of propanol is
Since, mass of
Therefore, mass of
The heat of combustion of propanol is
Substitute the value of heat of combustion of propanol and its mass in equation (1).
Isopropanol (
According to the above reaction,
Molar mass of isopropanol is
Since, mass of
Therefore, mass of
The heat of combustion of isopropanol is
Substitute the value of heat of combustion of isopropanol and its mass in equation (1).
The fuel value of diethyl ether is
The fuel value of diethyl ether is
Want to see more full solutions like this?
Chapter 13 Solutions
Chemistry
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





