
ORGANIC CHEMISTRY-PRINT COMPANION (LL)
4th Edition
ISBN: 9781119659594
Author: Klein
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Question
Chapter 13, Problem 73IP
Interpretation Introduction
Interpretation: The mechanism for the given transformation needs to be provided and stereochemistry needs to be explained.
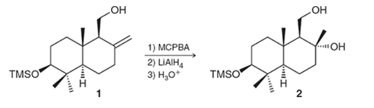
Concept Introduction:
In the presence of MCPBA, the formation of
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
3. Provide a mechanism for the following transformation:
Ph CI
(2 eq.)
2 eq. NaCN
4. Provide a mechanism for the following transformation:
S
H₂O
NC CN
Ph
Ph
OH
of
OH
The last step in the synthesis of β-vetivone, a major constituent of vetiver, a perennial grass found in tropical and subtropical regions of the world, involves treatment of C with CH3Li to form an intermediate X, which forms β-vetivone with aqueous acid. Identify the structure of X and draw a mechanism for converting X to β-vetivone.
A key step in the synthesis of β-vetivone, a major constituent of vetiver, a perennial grass found in tropical and subtropical regions of the world, involved the reaction of compound A and dihalide B with two equivalents of LDA to form C. Draw a stepwise mechanism for this reaction. β-Vetivone contains a spiro ring system—that is, two rings that share a single carbon atom.
Chapter 13 Solutions
ORGANIC CHEMISTRY-PRINT COMPANION (LL)
Ch. 13.2 - Prob. 1LTSCh. 13.2 - Prob. 1PTSCh. 13.2 - Prob. 2PTSCh. 13.2 - Prob. 3ATSCh. 13.4 - Prob. 4CCCh. 13.5 - Prob. 2LTSCh. 13.5 - Prob. 5PTSCh. 13.5 - Prob. 6ATSCh. 13.5 - Prob. 7CCCh. 13.5 - Prob. 8CC
Ch. 13.5 - Prob. 9CCCh. 13.6 - Prob. 10CCCh. 13.7 - Prob. 11CCCh. 13.7 - Prob. 12CCCh. 13.8 - Prob. 3LTSCh. 13.8 - Prob. 13PTSCh. 13.8 - Prob. 14ATSCh. 13.9 - Prob. 15CCCh. 13.10 - Prob. 4LTSCh. 13.10 - Prob. 17ATSCh. 13.10 - Prob. 5LTSCh. 13.10 - Prob. 19ATSCh. 13.11 - Prob. 20CCCh. 13.12 - Prob. 6LTSCh. 13.12 - Prob. 7LTSCh. 13 - Prob. 26PPCh. 13 - Prob. 27PPCh. 13 - Prob. 28PPCh. 13 - Prob. 29PPCh. 13 - Prob. 30PPCh. 13 - Prob. 31PPCh. 13 - Prob. 32PPCh. 13 - Prob. 33PPCh. 13 - Prob. 34PPCh. 13 - Prob. 35PPCh. 13 - Prob. 36PPCh. 13 - Prob. 37PPCh. 13 - Prob. 38PPCh. 13 - Prob. 39PPCh. 13 - Prob. 40PPCh. 13 - Prob. 41PPCh. 13 - Prob. 42PPCh. 13 - Prob. 43PPCh. 13 - Prob. 44PPCh. 13 - Prob. 45PPCh. 13 - Prob. 46ASPCh. 13 - Prob. 47ASPCh. 13 - Prob. 48ASPCh. 13 - Prob. 49ASPCh. 13 - Prob. 50ASPCh. 13 - Prob. 51ASPCh. 13 - Prob. 52ASPCh. 13 - Prob. 53ASPCh. 13 - Prob. 54IPCh. 13 - Prob. 59IPCh. 13 - Prob. 60IPCh. 13 - Prob. 61IPCh. 13 - Prob. 62IPCh. 13 - Prob. 63IPCh. 13 - Prob. 64IPCh. 13 - Prob. 65IPCh. 13 - Prob. 66IPCh. 13 - Prob. 69IPCh. 13 - Prob. 70IPCh. 13 - Prob. 71IPCh. 13 - Prob. 72IPCh. 13 - Prob. 73IPCh. 13 - Prob. 74IPCh. 13 - Prob. 77CPCh. 13 - Prob. 79CPCh. 13 - Prob. 80CP
Knowledge Booster
Similar questions
- Show the products from reaction of p-bromoaniline with the following reagents: (a) CH3I (excess) (b) HCl (c) HNO2, H2SO4 (d) CH3COCl (e) CH3MgBr (f) CH3CH2Cl, AlCl3 (g) Product of (c) with CuCl, HCl (h) Product of (d) with CH3CH2Cl, AlCl3arrow_forwardTreatment of an aldehyde or ketone with cyanide ion (-:C=N), followed by protonation of the tetrahedral alkoxide ion intermediate, gives a cyanohydrin. Show the structure of the cyanohydrin obtained from cyclohexanone.arrow_forwardIdentify the reagent that accomplish the transformation seen below: 1) 03 2) Zn/H₂O H₂O/H2SO4 (cat.) OH₂/Pd O 1) BH3 2) H₂O2/NaOH / H₂O m-CPBA (meta-chloroperoxybenzoic acid) HOarrow_forward
- Propose a plausible mechanism for the following transformation: 1) Excess MeMgBr 2) H₂O HOarrow_forwardWhen 2-methylcyclohexanone is treated with pyrrolidine, two isomeric enamines areformed.Why is enamine A with the less substituted double bond the thermodynamicallyfavored product? (You will find it helpful to examine the models of these twoenamines.)arrow_forwardPropose a mechanism for the following reaction:arrow_forward
- Identify the reagents (a–h) needed to carry out each reaction.arrow_forwardDraw a stepwise mechanism for the following reactions, two steps in R. B. Woodward’s classic synthesis of reserpine in 1958. Reserpine, which is isolated from the extracts of the Indian snakeroot Rauwola serpentina Benth, was used at one time to manage mild hypertension associated with anxiety.arrow_forwardPropose mechanisms for the following reactions. (b) OH H3PO4 CH₂OH heat H2SO4 heat (c) OH H2SO4 heat (d) OH H2SO4 heat CH2 CH3 8.5.0 + + +arrow_forward
- Propose a plausible mechanism for the following transformation: 1) Excess MeMgBr 2) H₂O HO OHarrow_forward1arrow_forwardReaction of 5,5-dimethoxypentan-2-one with methylmagnesium iodide followed by treatment with aqueous acid forms cyclic hemiacetal Y. Draw a stepwise mechanism that illustrates how Y is formed.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning